2.7/3.5 mm Locking Medial Distal Humerus Plates Specification, Uses, Sizes & Surgical Instruments.

2.7/3.5 mm Locking Medial Distal Humerus Plates Specification
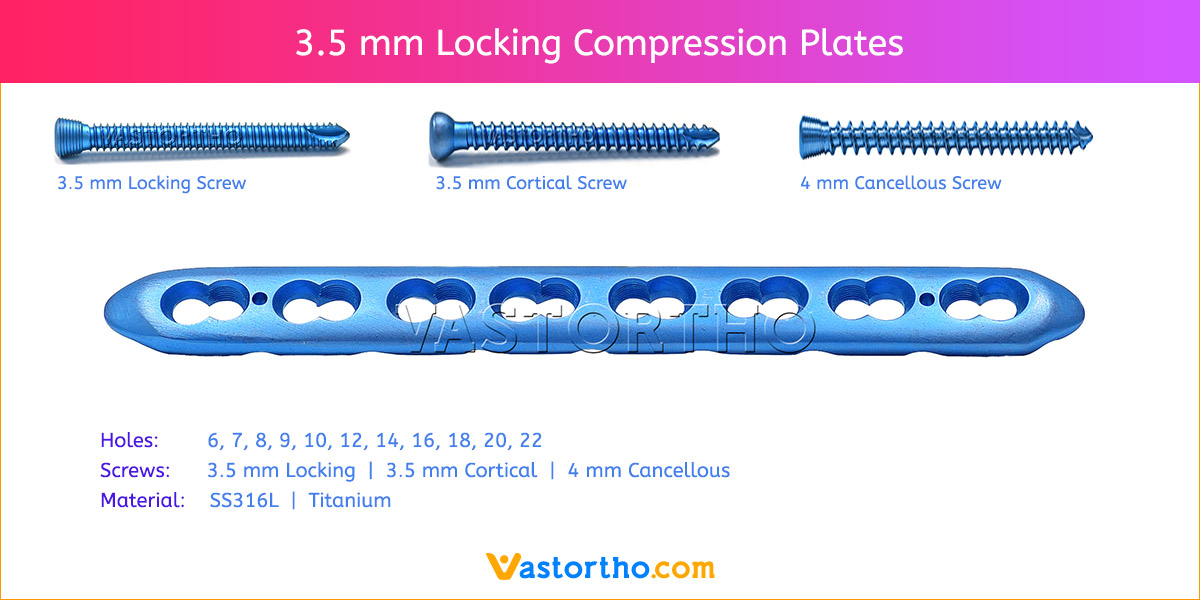
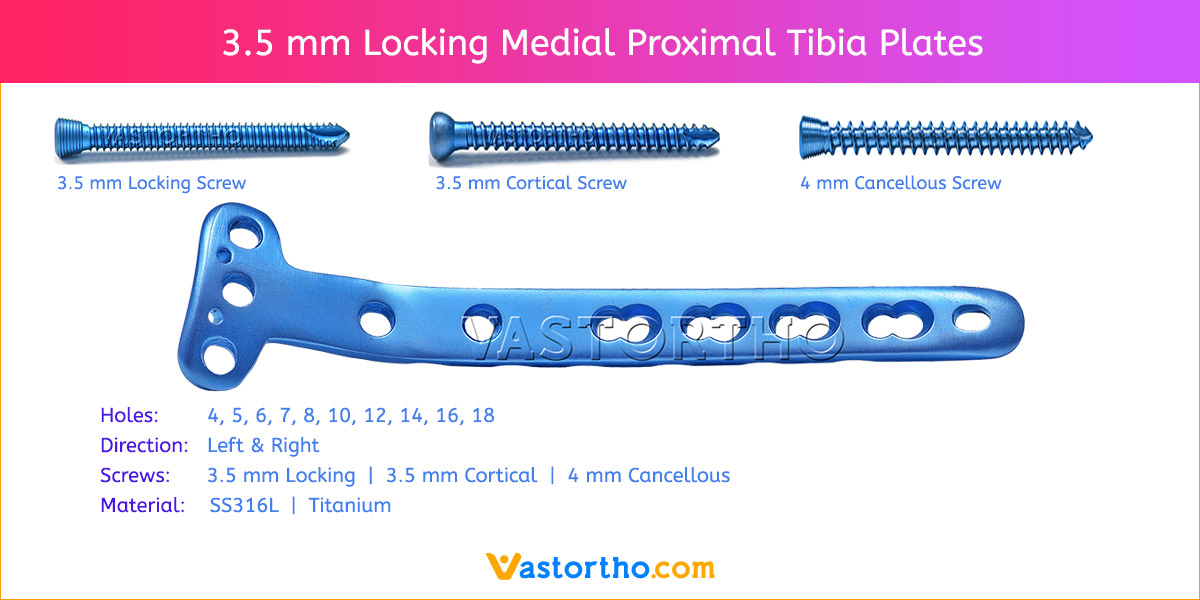
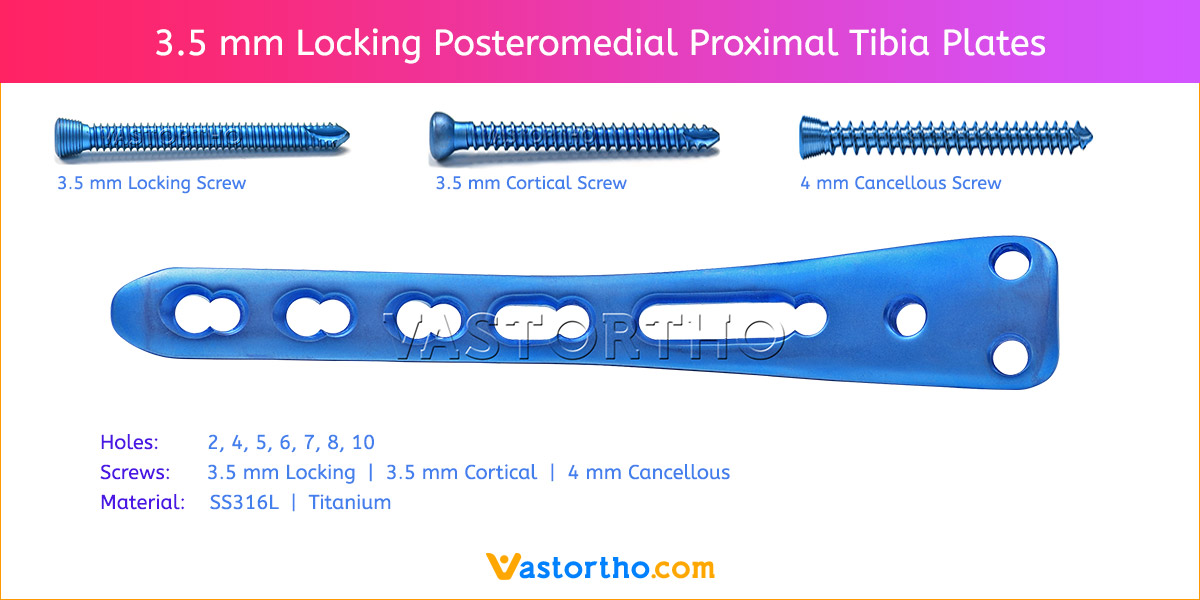
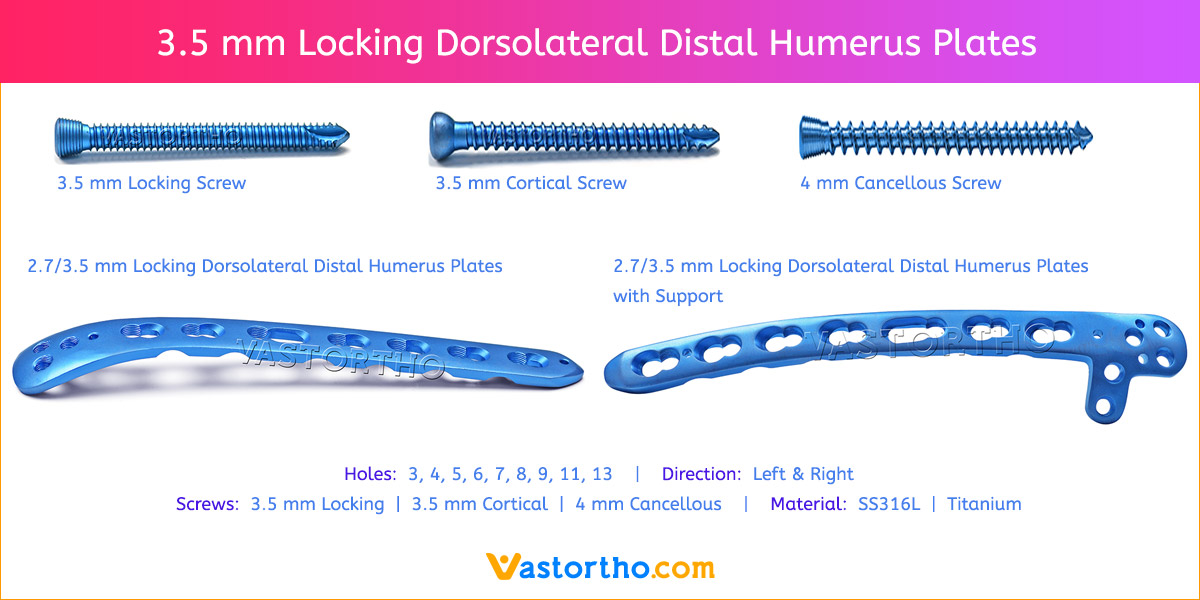
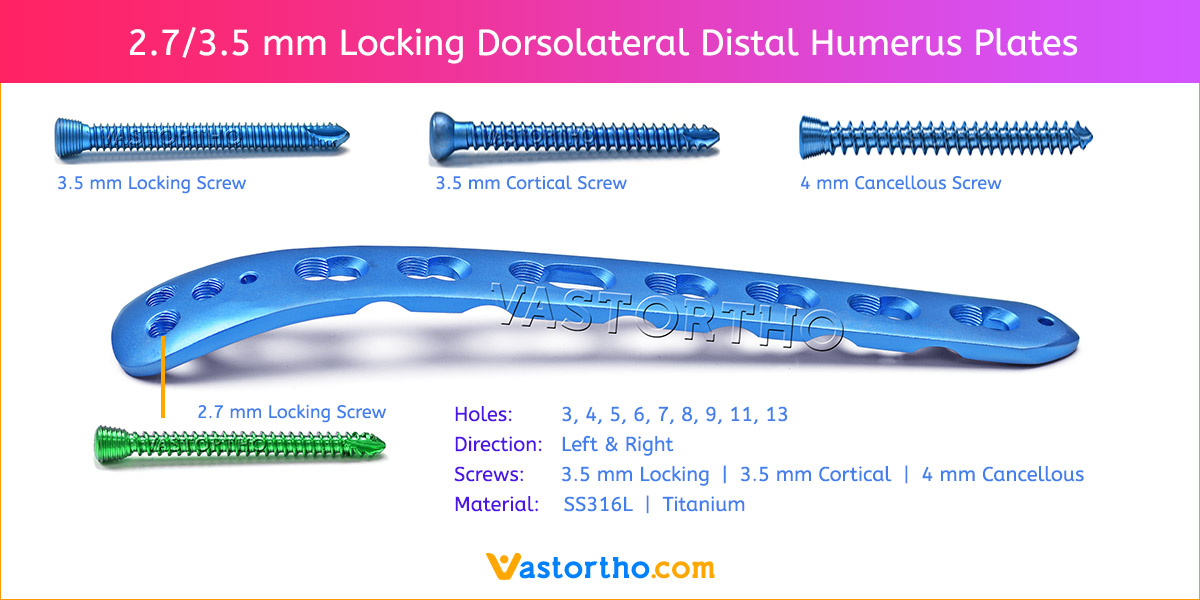
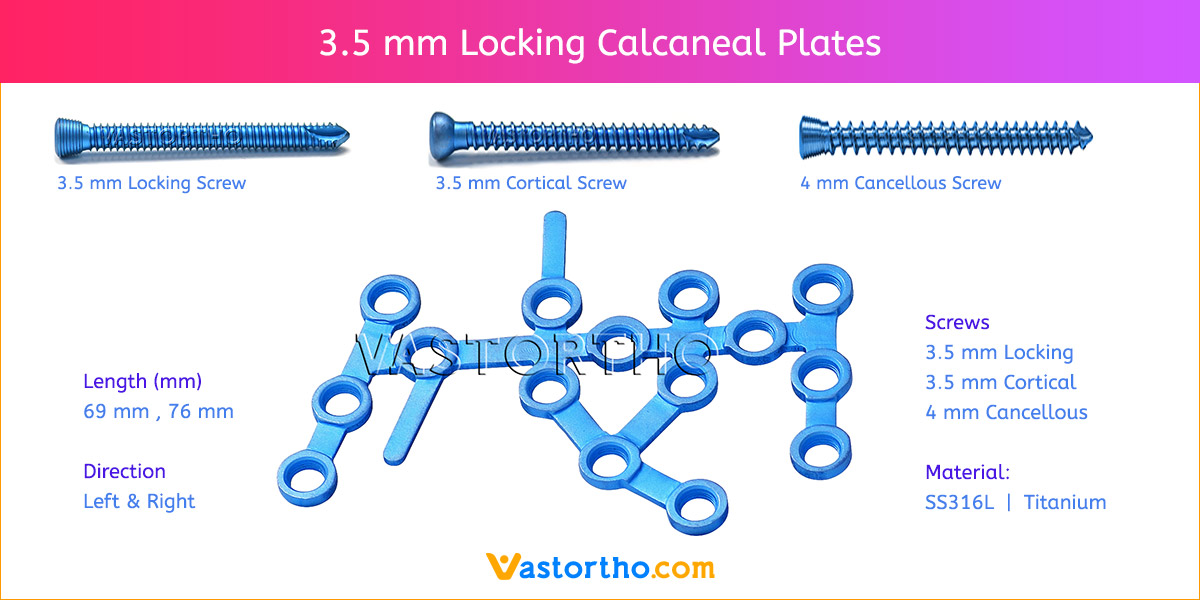
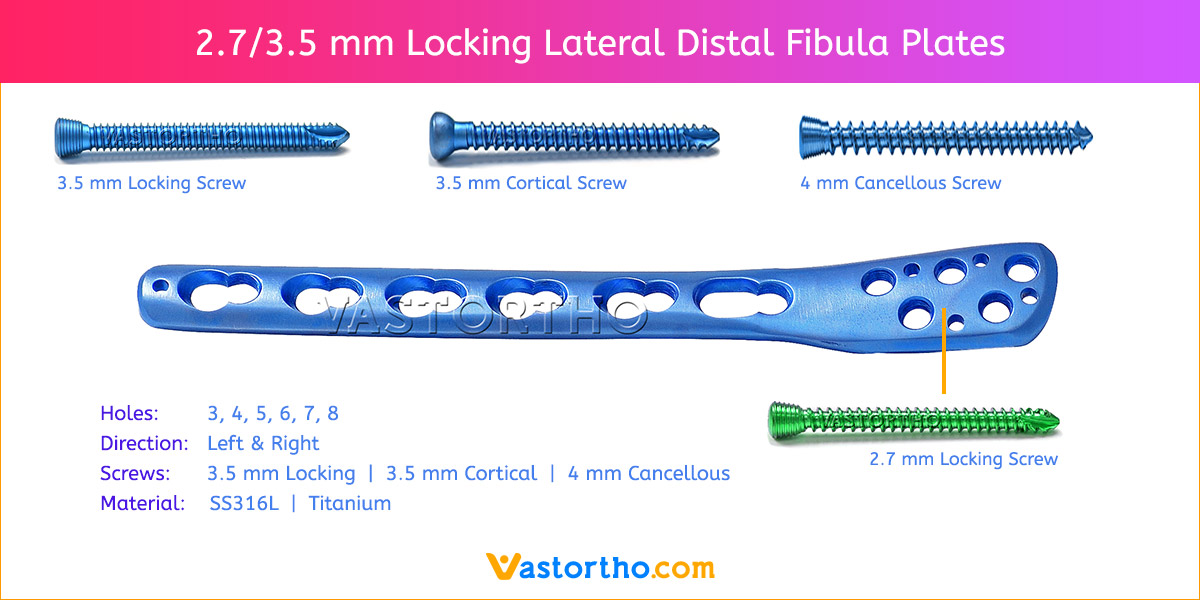
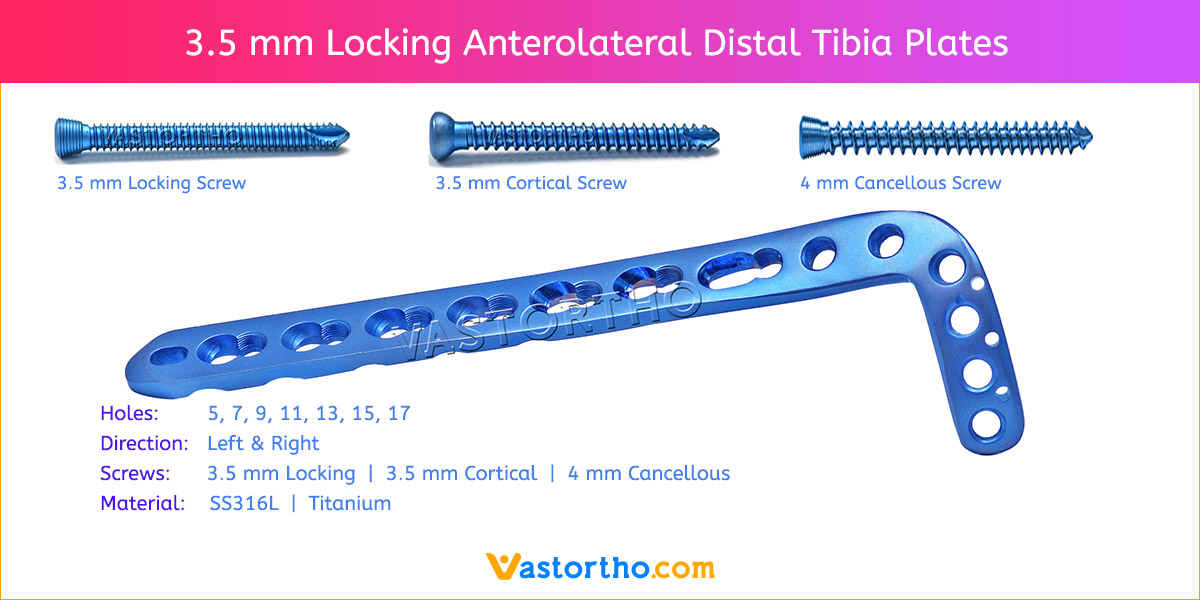
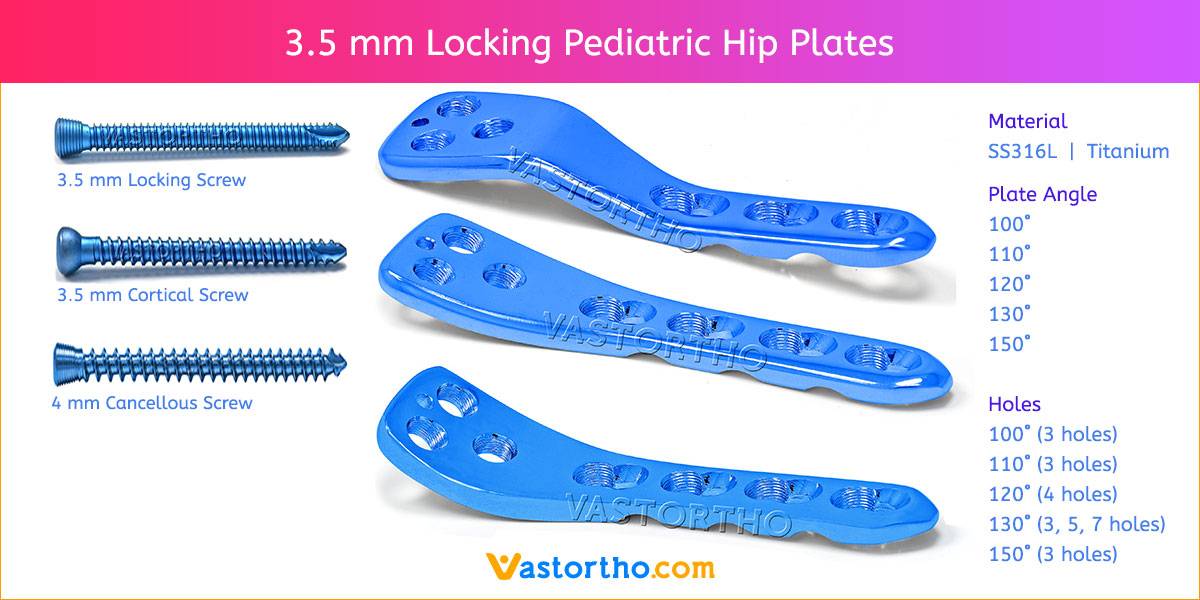
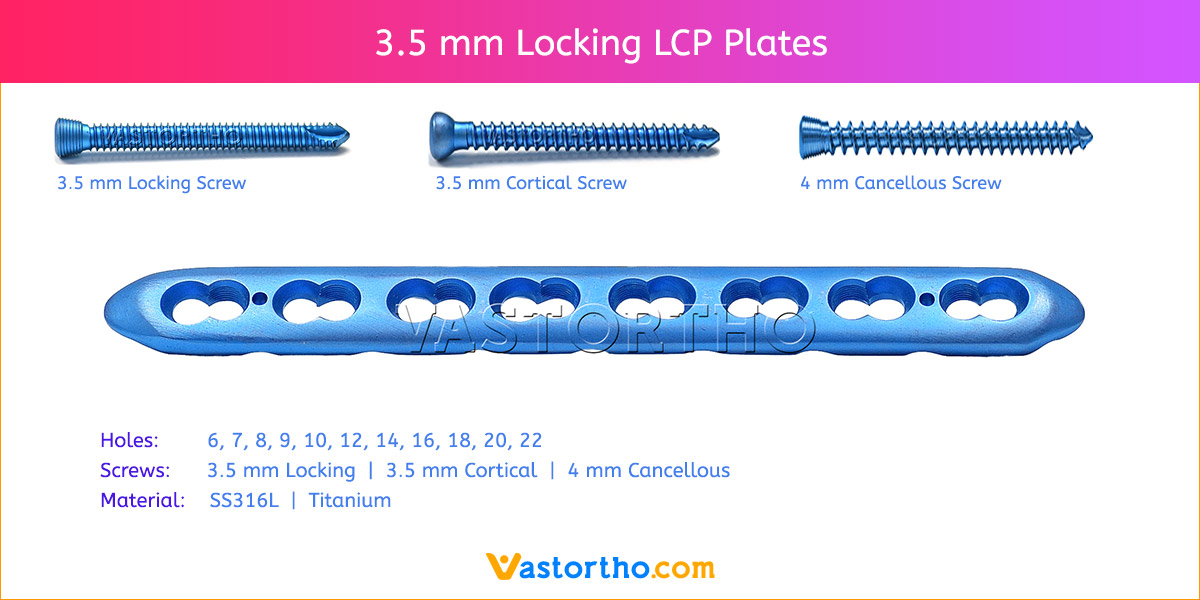
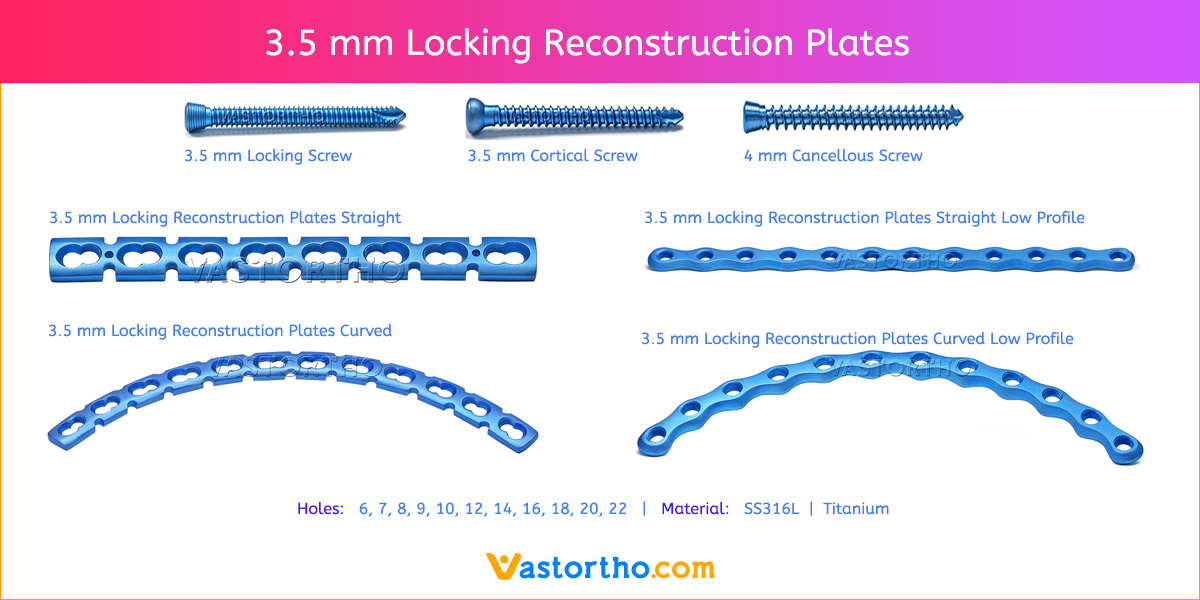
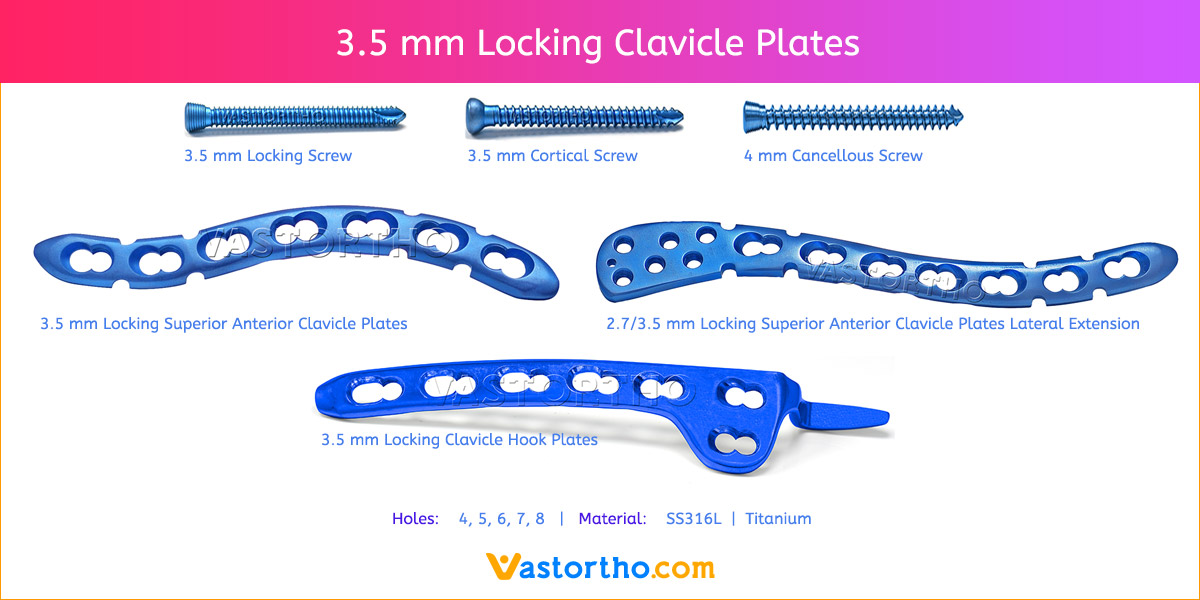
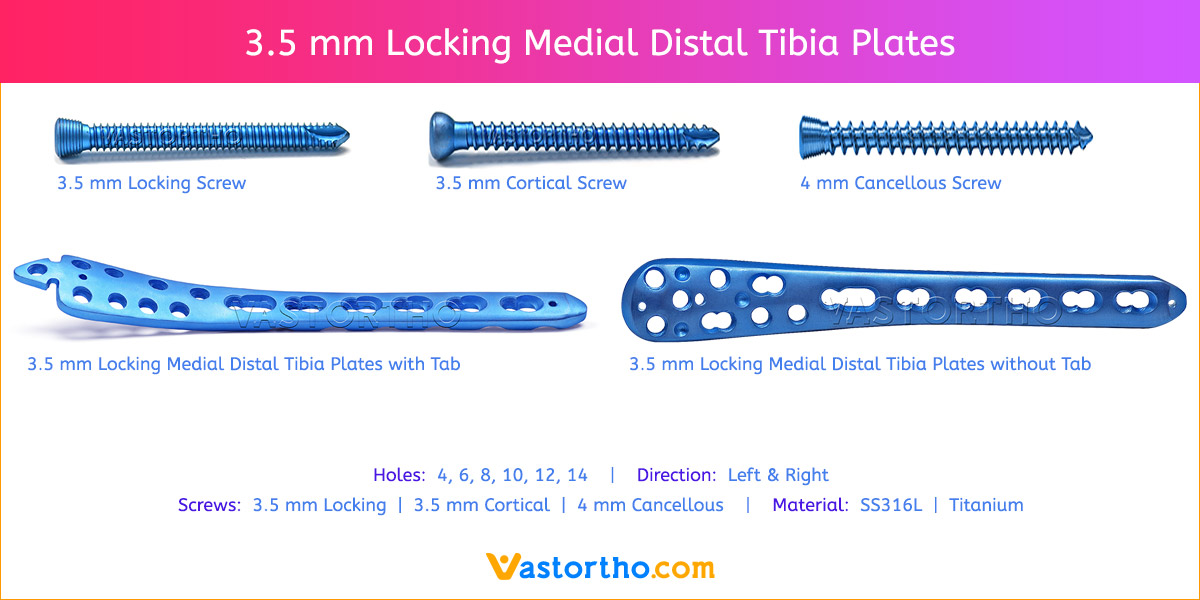
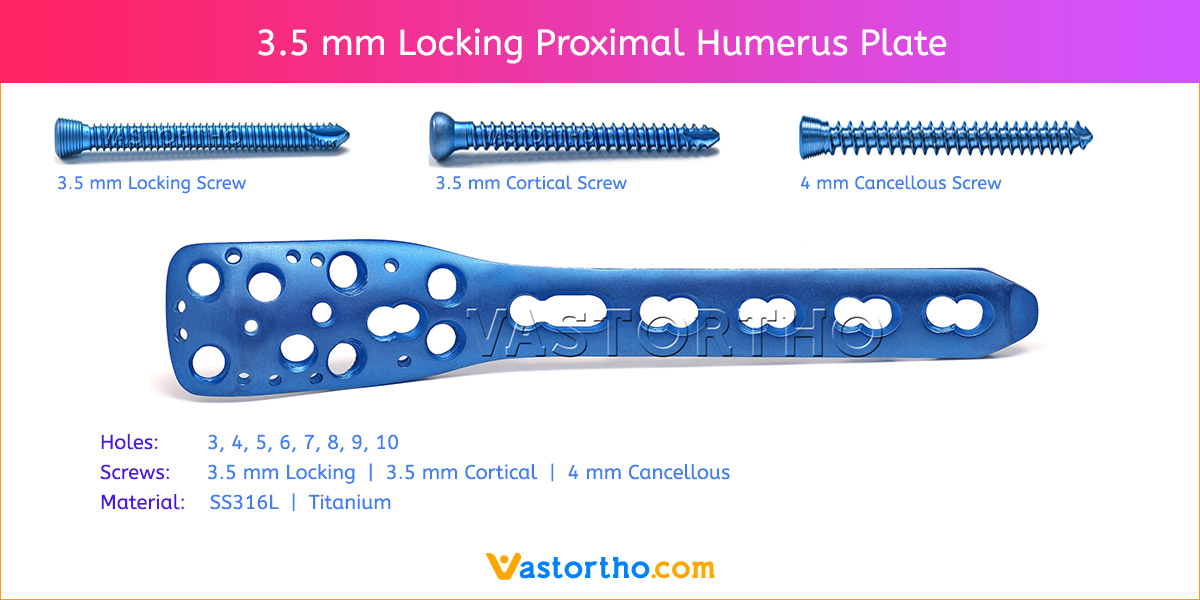
- Plates available holes are 3, 4, 5, 6, 7, 8, 9, 11 and 13..
- Plates available for Left and Right both direction
- Plate has combi holes and round holes. Combi holes allow fixation with locking screws in the threaded section and cortex screws in the dynamic compression unit section for compression.
- The shaft holes accept 3.5 mm locking screws in the threaded portion or 3.5 mm cortical screws or 4 mm cancellous screws in the compression portion. Distal locking holes in plate head accept 2.7 mm locking screws.
- 2.7/3.5 mm Locking Medial Distal Humerus Plates allow implant placement to address the individual fracture pattern.
- Limited-contact surface reduces bone-to-plate contact and helps to preserve the periosteal blood supply.
- Choice of different lengths of plate eliminates the need to cut plates.
- Pre-contoured plate to match anatomical shape.
- Available in both Titanium and Stainless steel.
- locking plate increases construct stability, decreases risk of screw back-out and subsequent loss of reduction. It also reduces the need for precise anatomic plate contouring and minimizes the risk of stripped screw holes.
- A complete Instruments Set is available for 2.7/3.5 mm Locking Medial Distal Humerus Plates. General Instruments are available for this plate such as Plate Bending Press, Plate Holding Forceps, Plate Bending Pliers, Bone Holding Forceps, Bone Elevators, Bone Cutter, Bone Nibbler, Depth Gauge, Sleeve, Screw Driver, Trocar Sleeve etc.
2.7/3.5 mm Locking Medial Distal Humerus Plates Uses
2.7/3.5 mm Locking Medial Distal Humerus Plates are indicated for intra-articular fractures of the distal humerus, comminuted supracondylar fractures, osteotomies, and nonunions of the distal humerus.