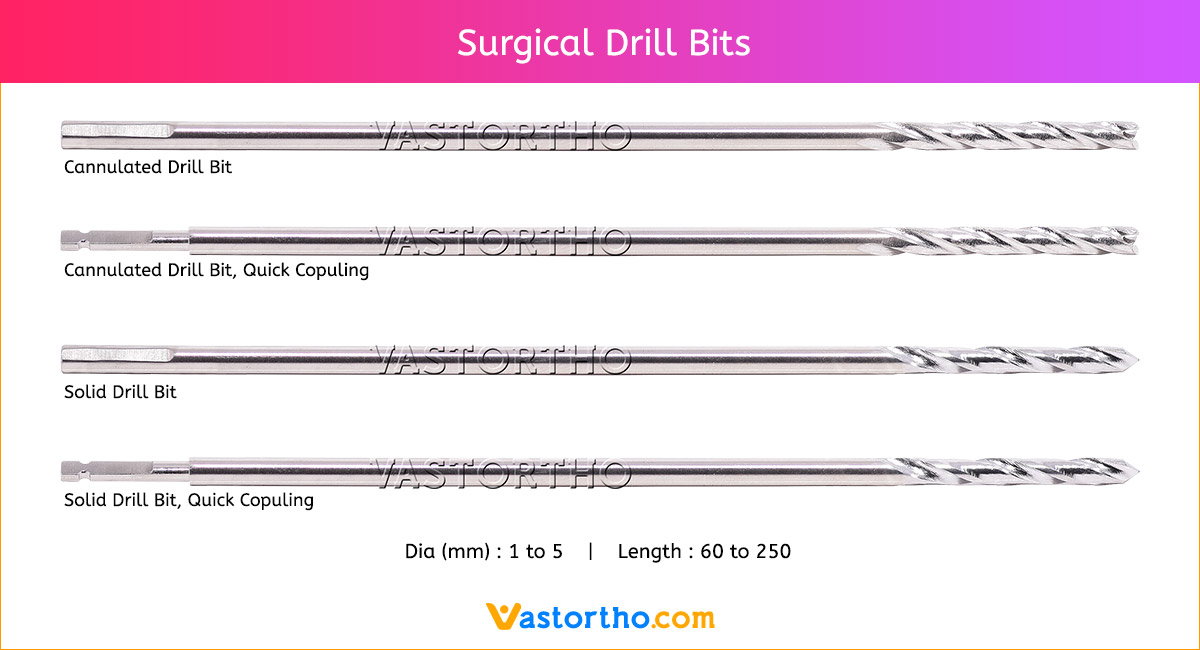
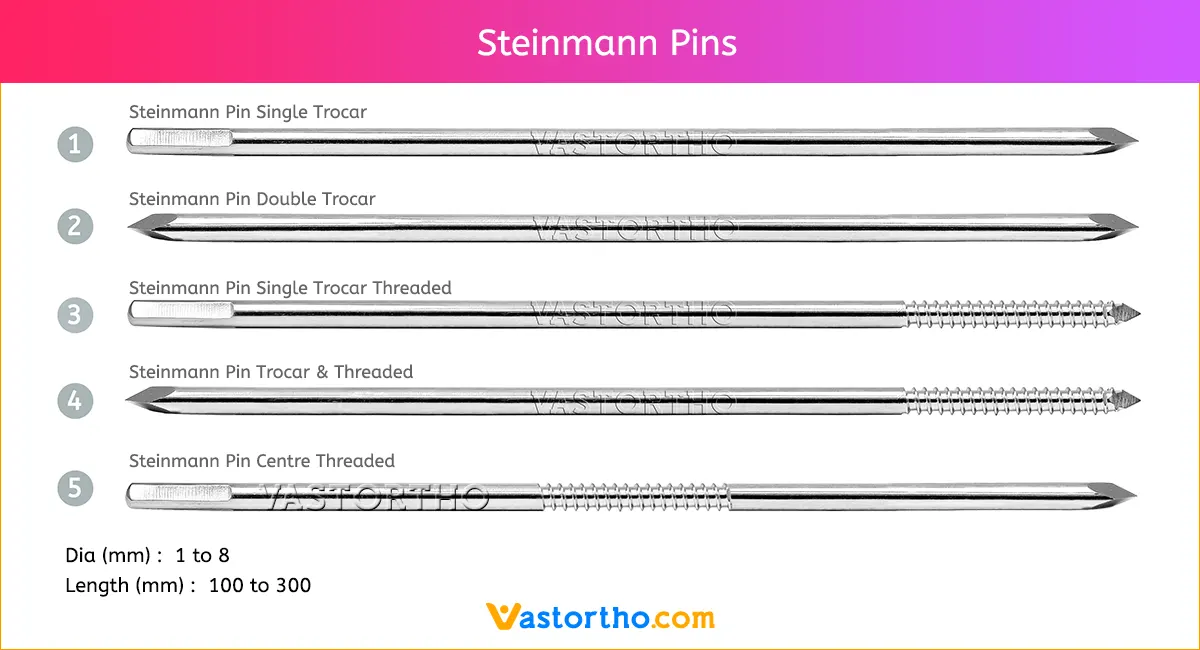
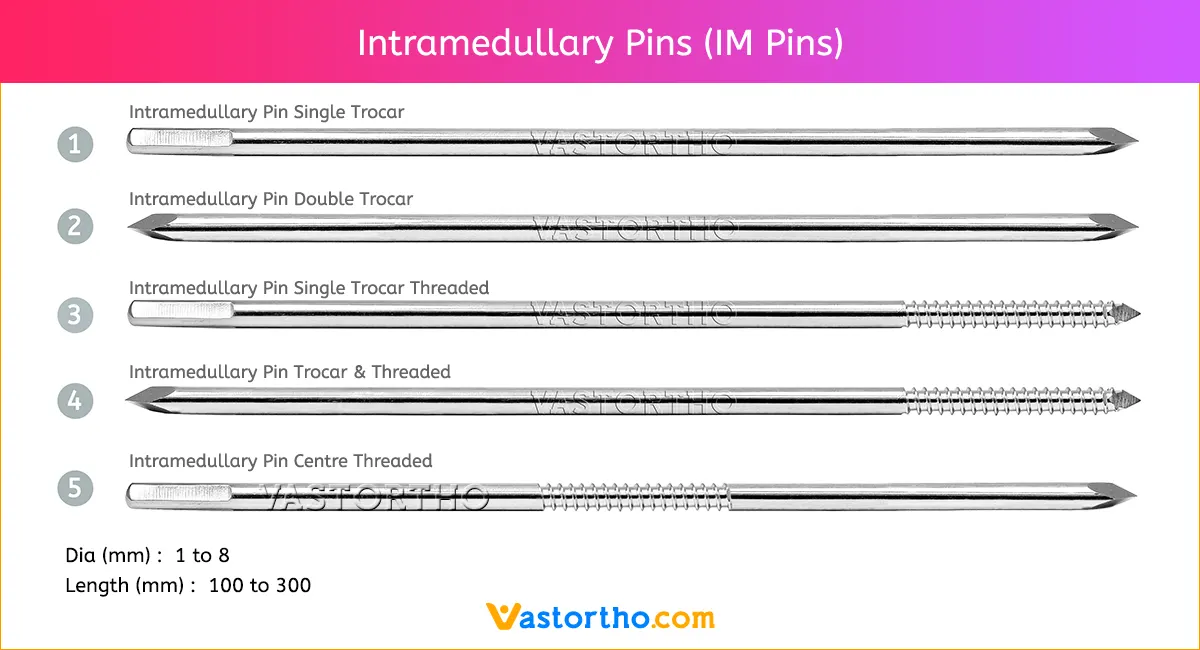
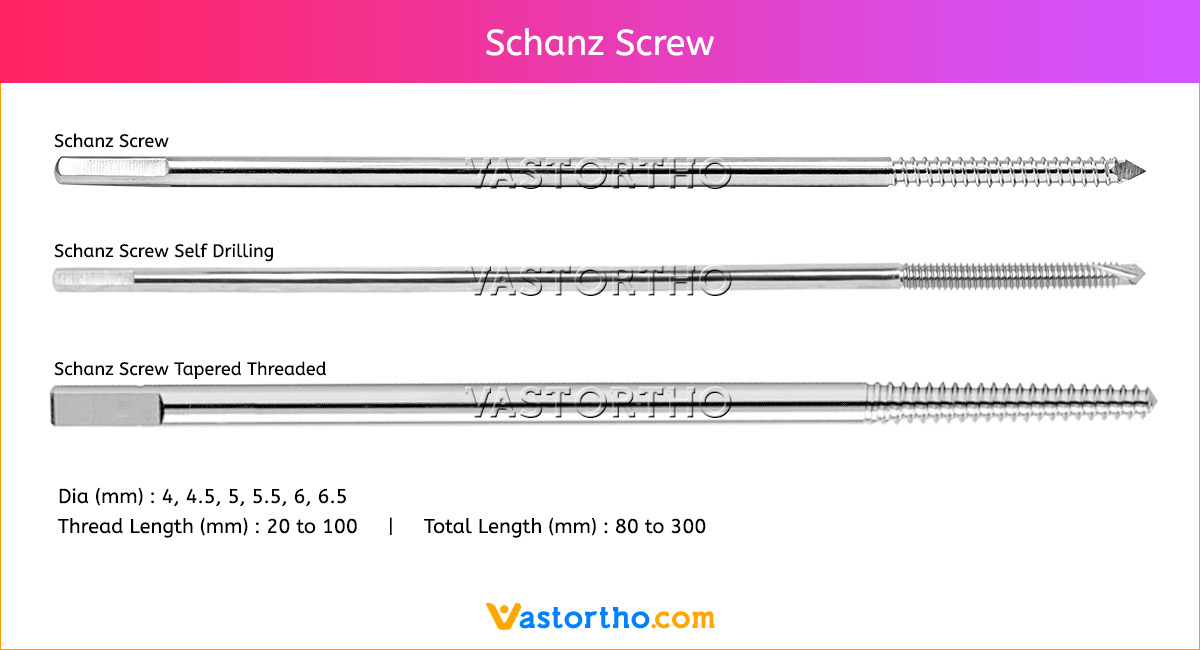
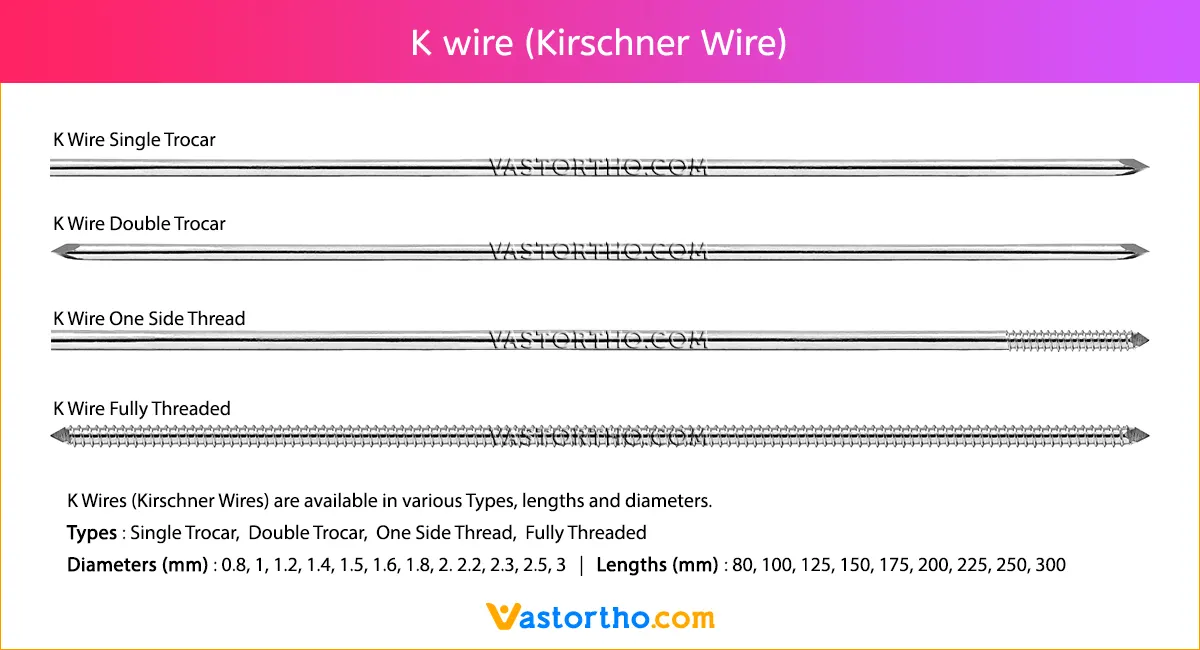
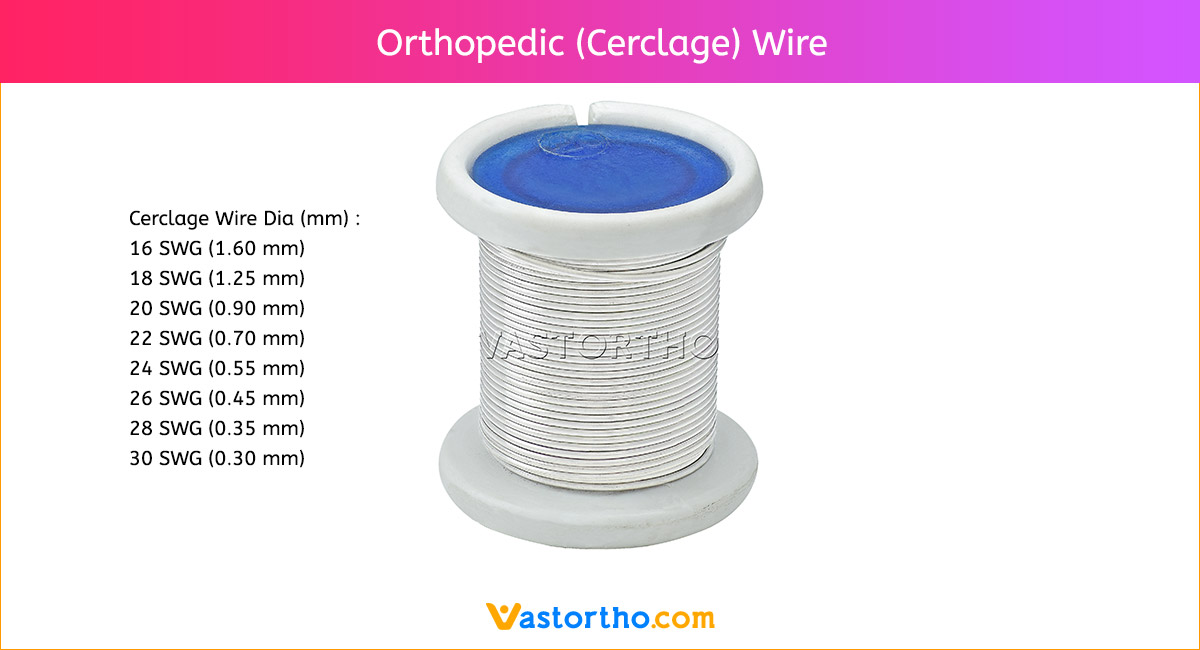
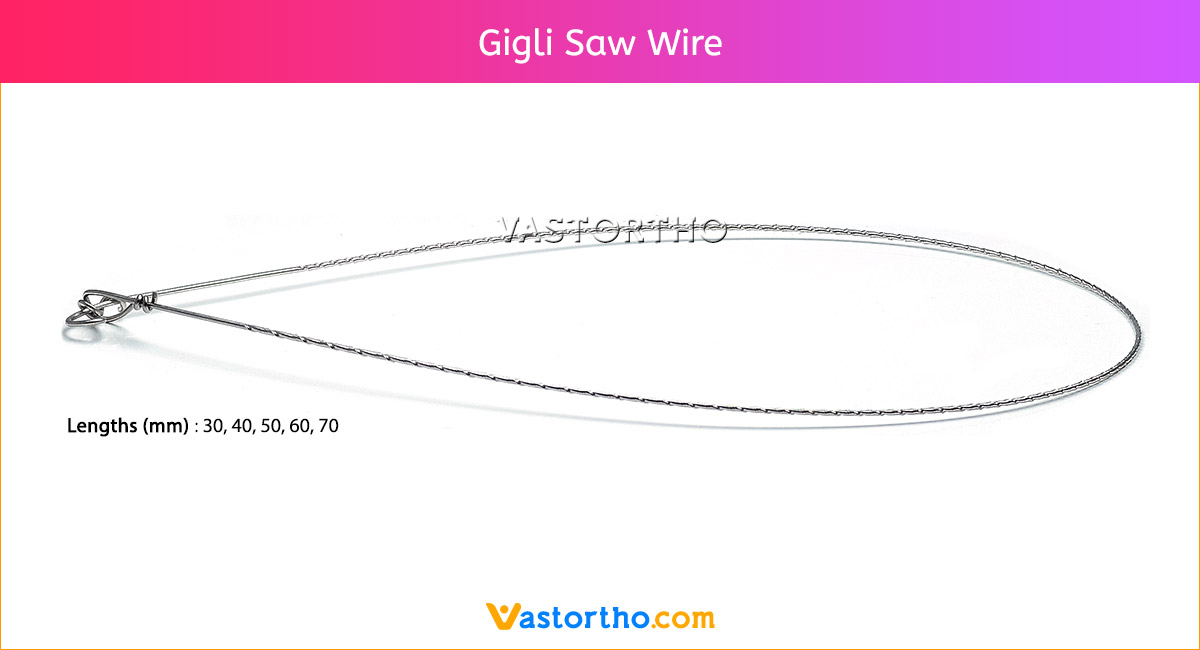
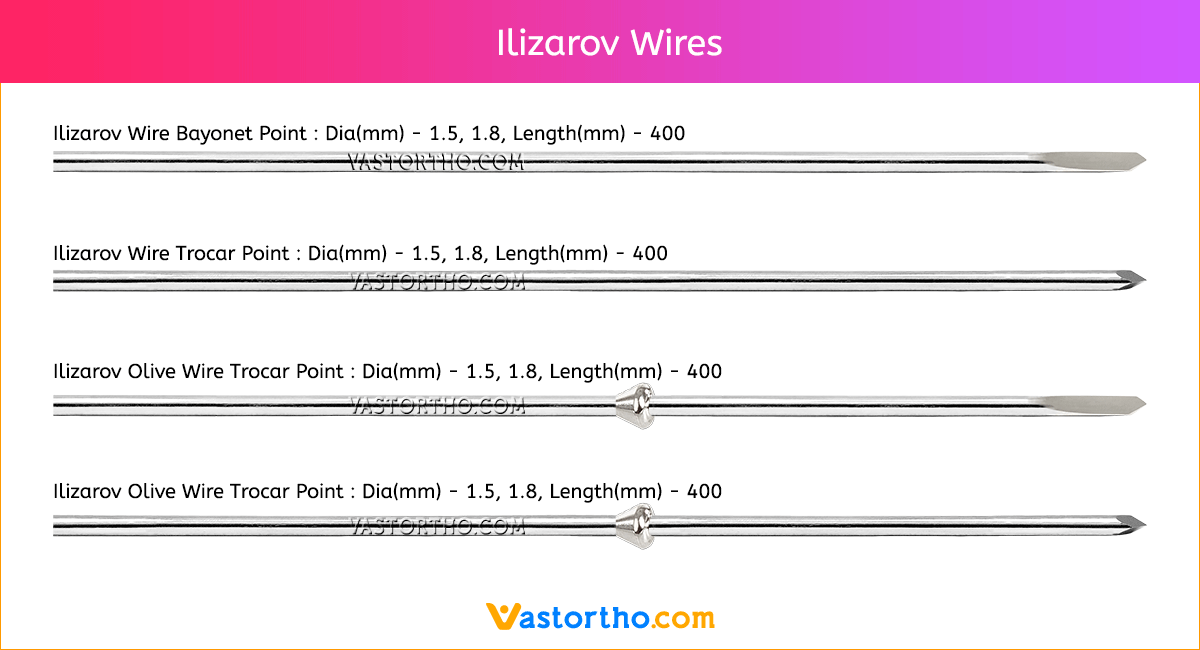
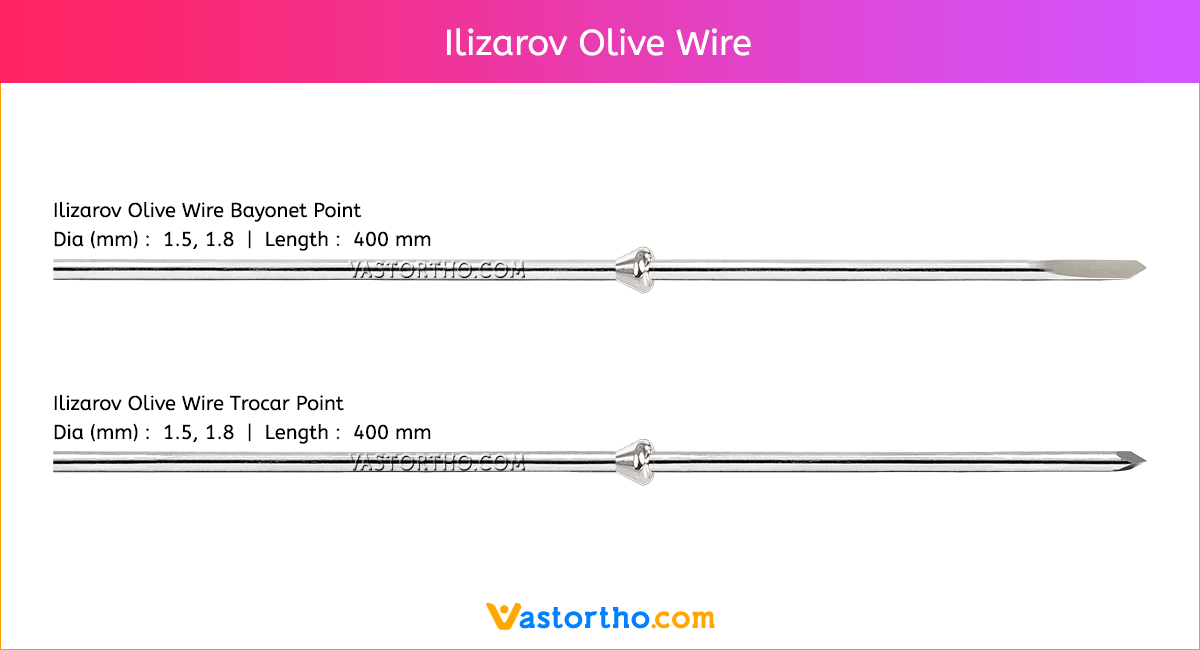
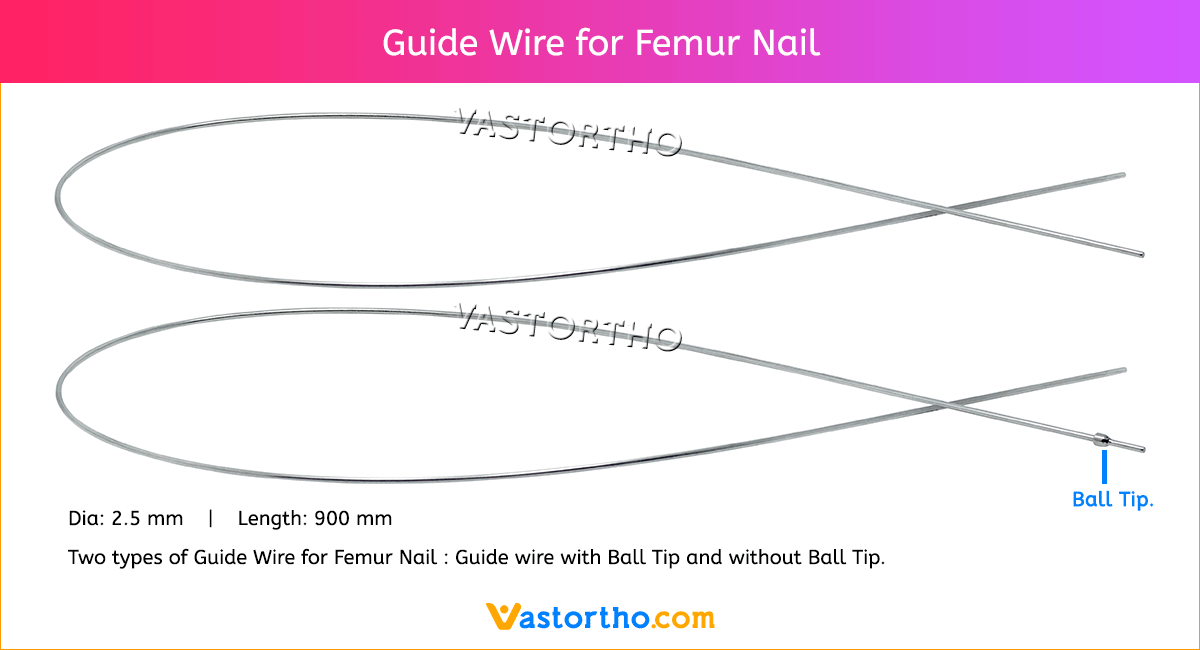
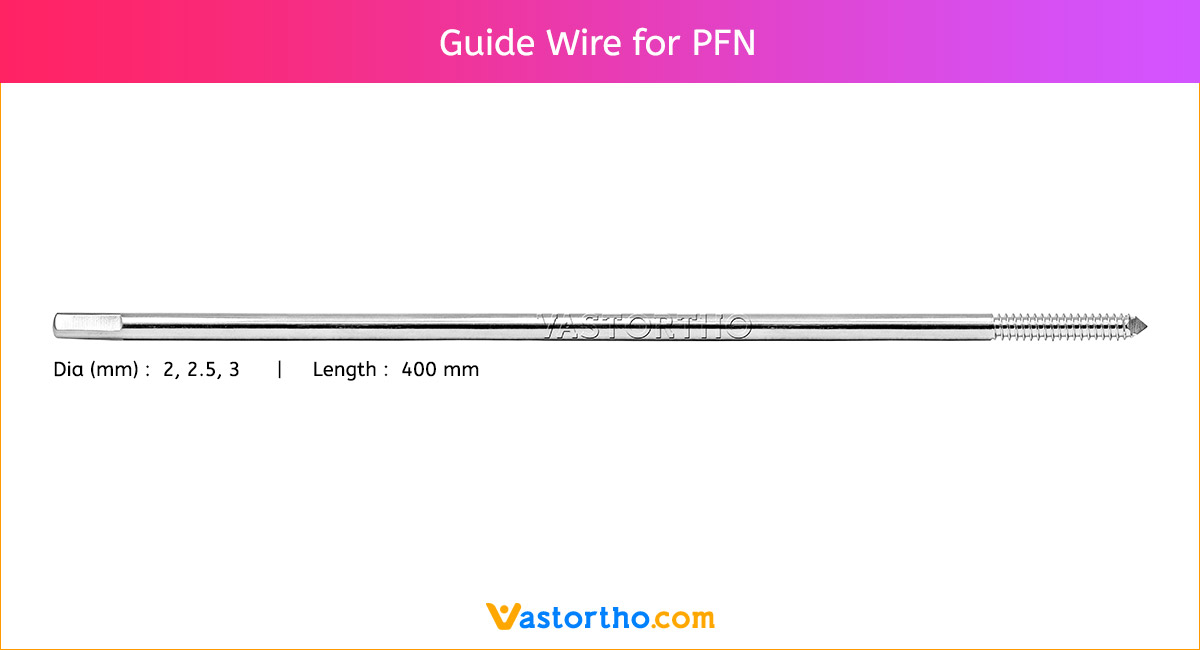
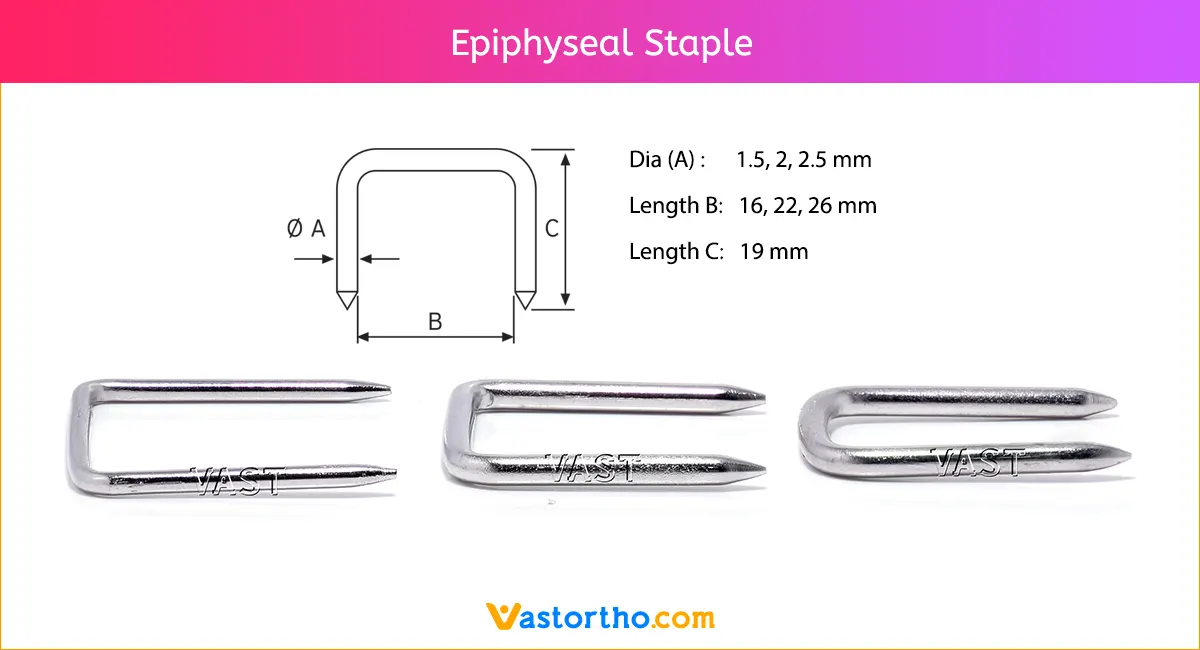
Guide Wire Tibia Specification, Uses, Sizes and Surgical Techniques.

A Guide Wire is a thin, flexible, medical wire inserted into the body to guide a larger instrument. Our Guide Wire Tibia is made from finest quality of SS 316L material to ensure highest quality. Guide Wire Tibia Diameter is 2.5 mm and length is 900 mm. There are two types of Guide Wire for Tibia nail: guide wire with ball tip and without ball tip.
Most nails are inserted over a guide wire. Reaming is always performed over a guide wire.
The Guide Wire Tibia is inserted under X-ray control.
In order to facilitate passing the guide wire across the fracture site, the fracture should be adequately aligned. It may be easier to pass the guide wire if the tip is angulated.
If the fracture ends cannot be sufficiently aligned for a guide wire to passed, an aiming device may be inserted into the proximal fragment (after reaming if necessary) to manipulate the proximal fragment so that the guide wire tibia can be passed into the distal fragment.
The correct length of the nail is determined by comparing a second guide wire of the same length to the one that has been inserted. The correct placement of the guide wire tibia in the distal canal should be assessed via image intensifier. The tip of the second guide wire must be positioned at the entry point in the bone.