Intramedullary Pin (IM Pin) Specification, Uses, Sizes and Surgical Techniques.
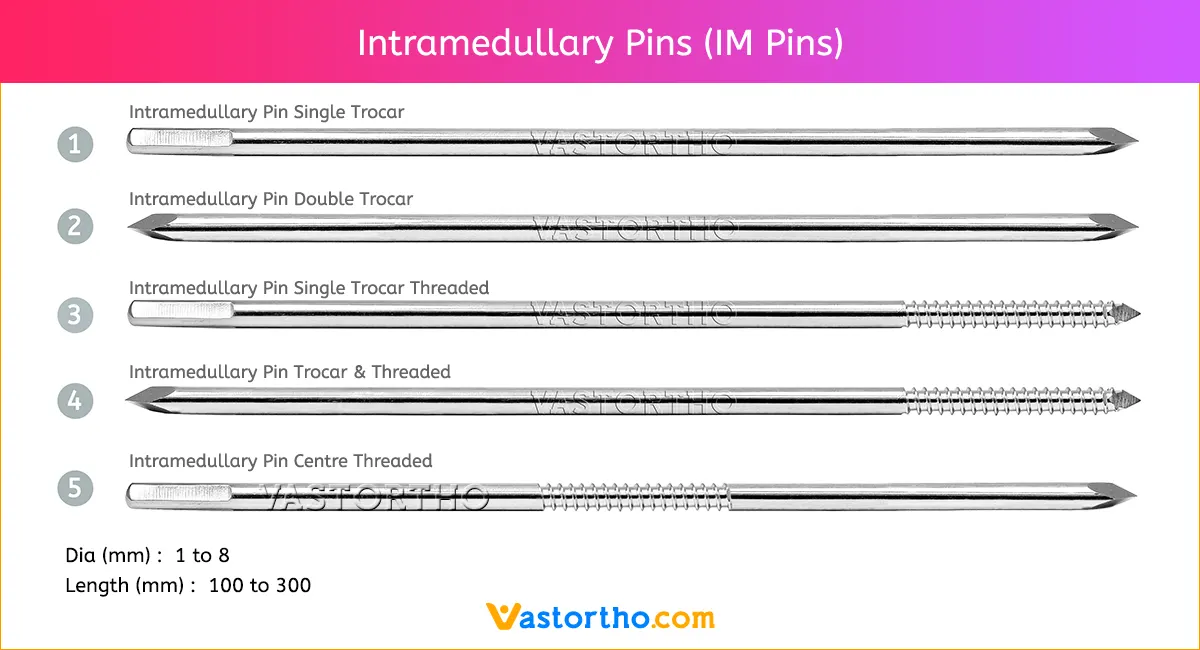
Intramedullary Pin (IM Pin) Specification
Intramedullary Pin (IM Pin) insertion remains the most commonly performed procedure in orthopedic practice. Although its use for definitive fracture treatment has steadily declined due to advent of newer implants and devices, it still finds application in treatment in fractures suffered by medically unfit and pediatric patients and for providing interim traction in almost all lower limb injuries as well as hip and knee pathologies.
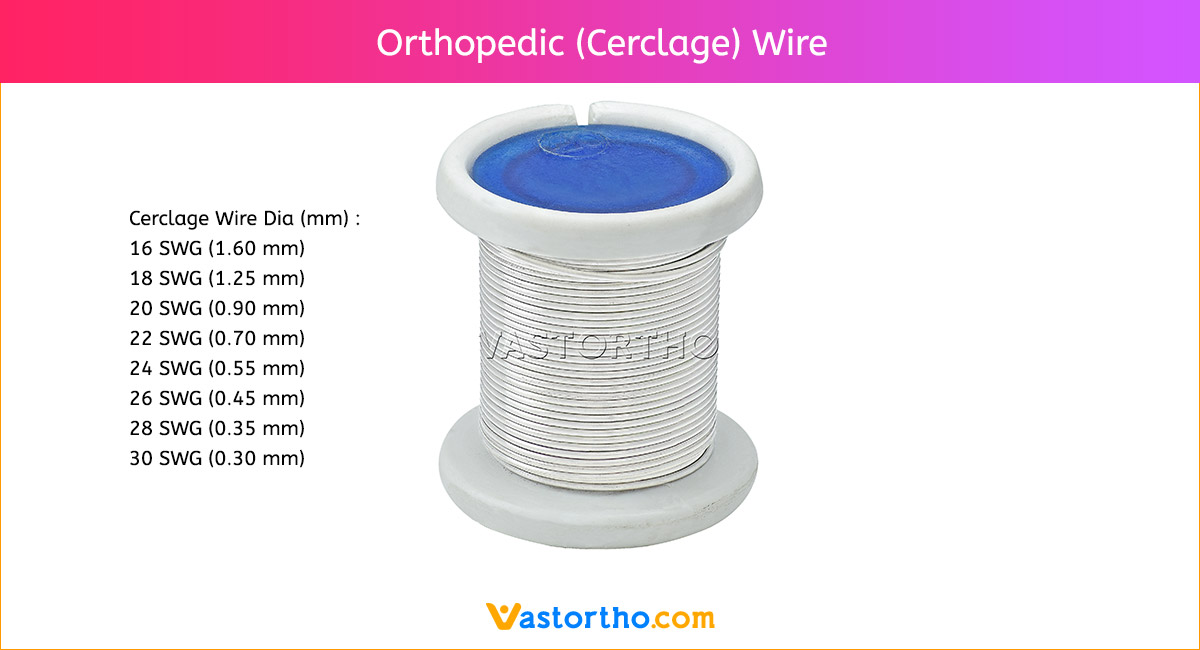
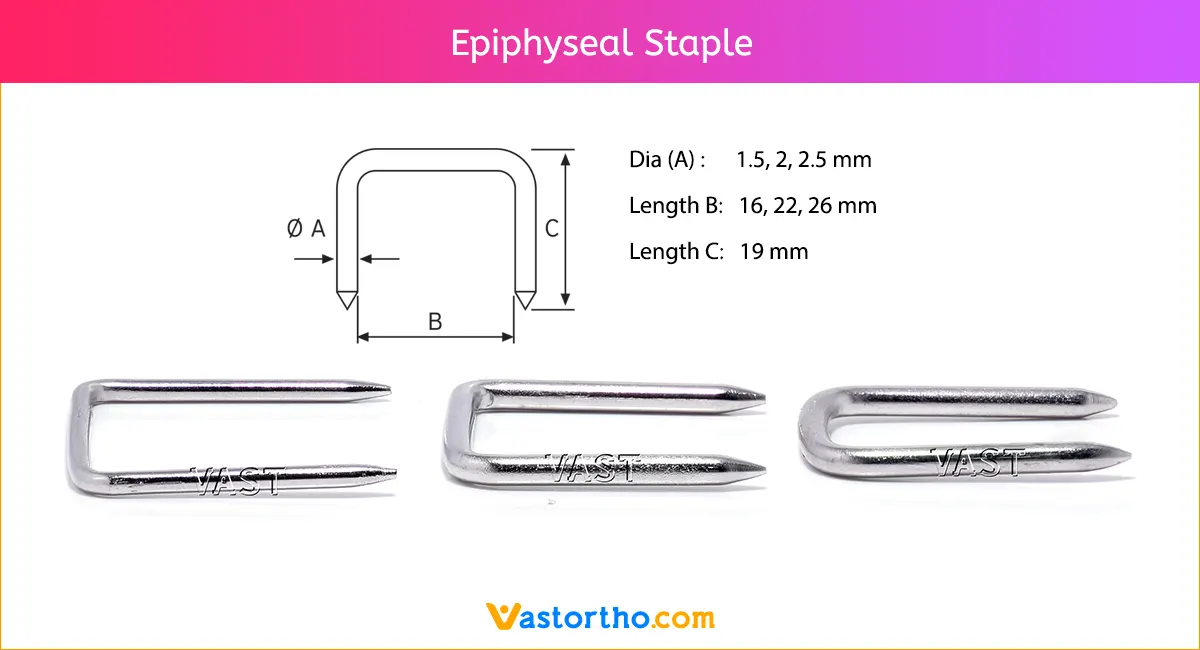
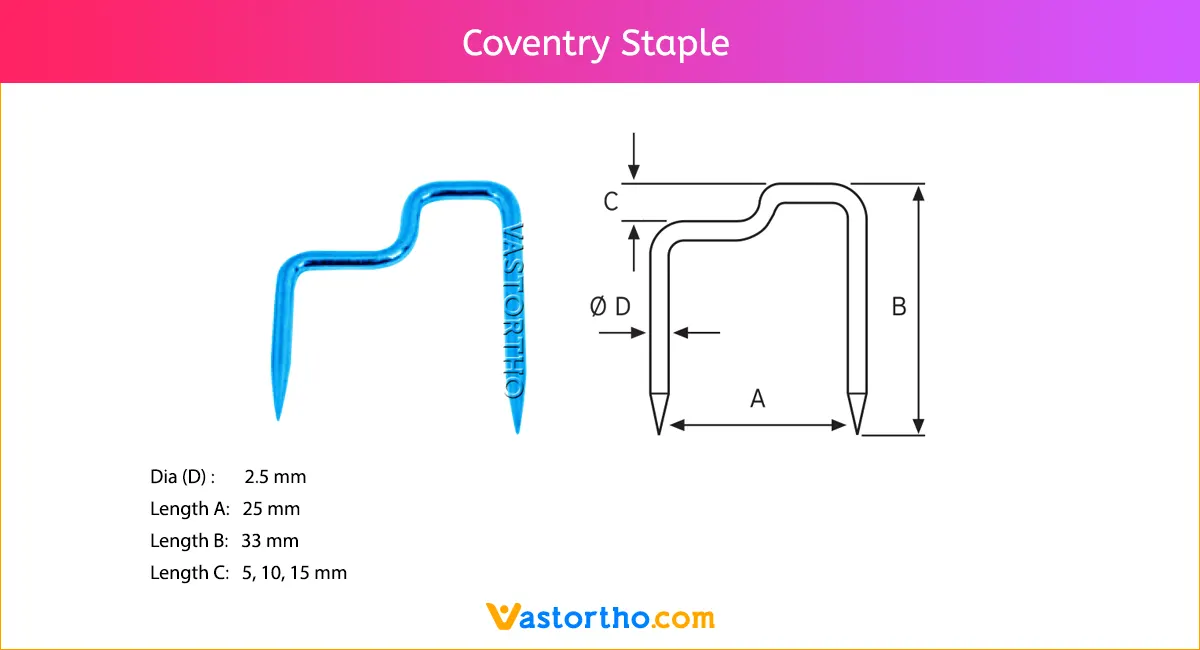
Intramedullary Pin (IM Pin) is composed of biocompatible materials such as stainless steel. They are smooth and cylindrical, with a sharp tip on one end and a hole or groove on the other.
Intramedullary Pin (IM Pin) Sizes
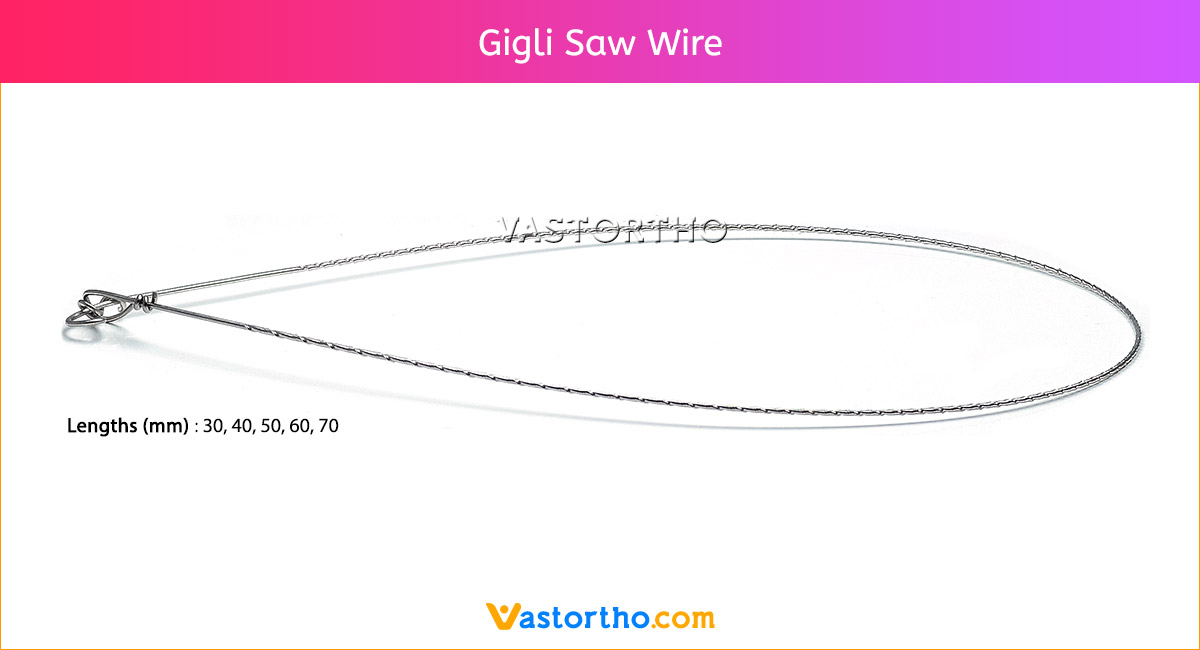
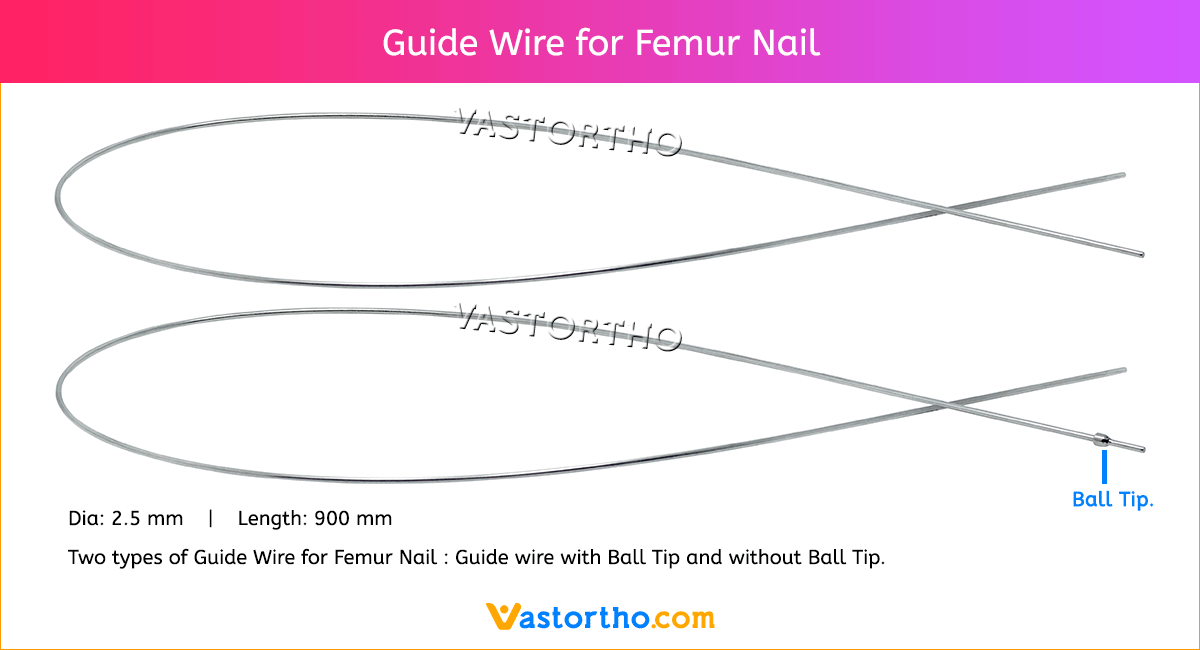
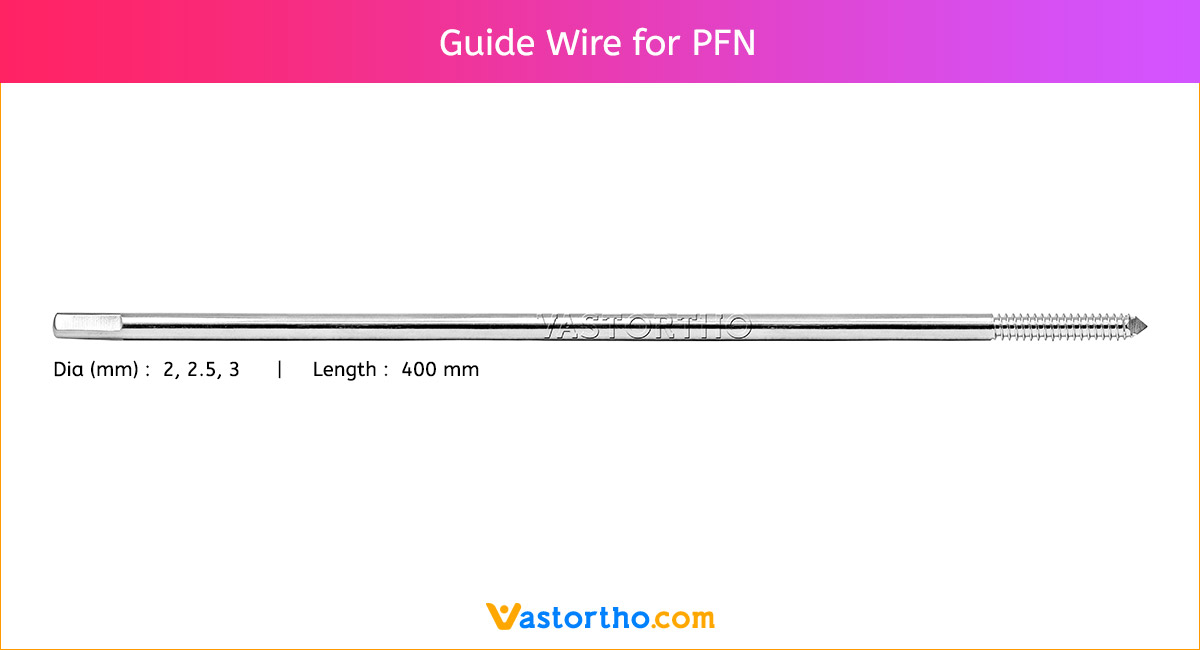
Intramedullary Pin (IM Pin) is a thin metal rod commonly used in orthopedic procedures for holding large bone fractures together. These pins function similarly to K Wire, but are larger in diameter.
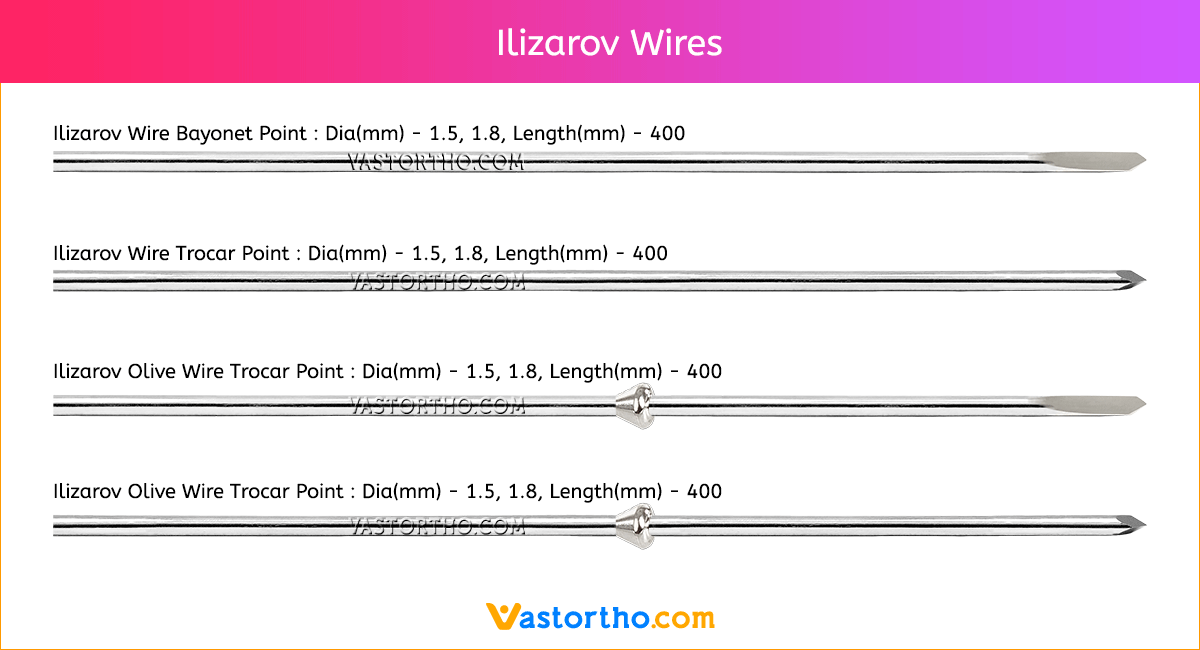
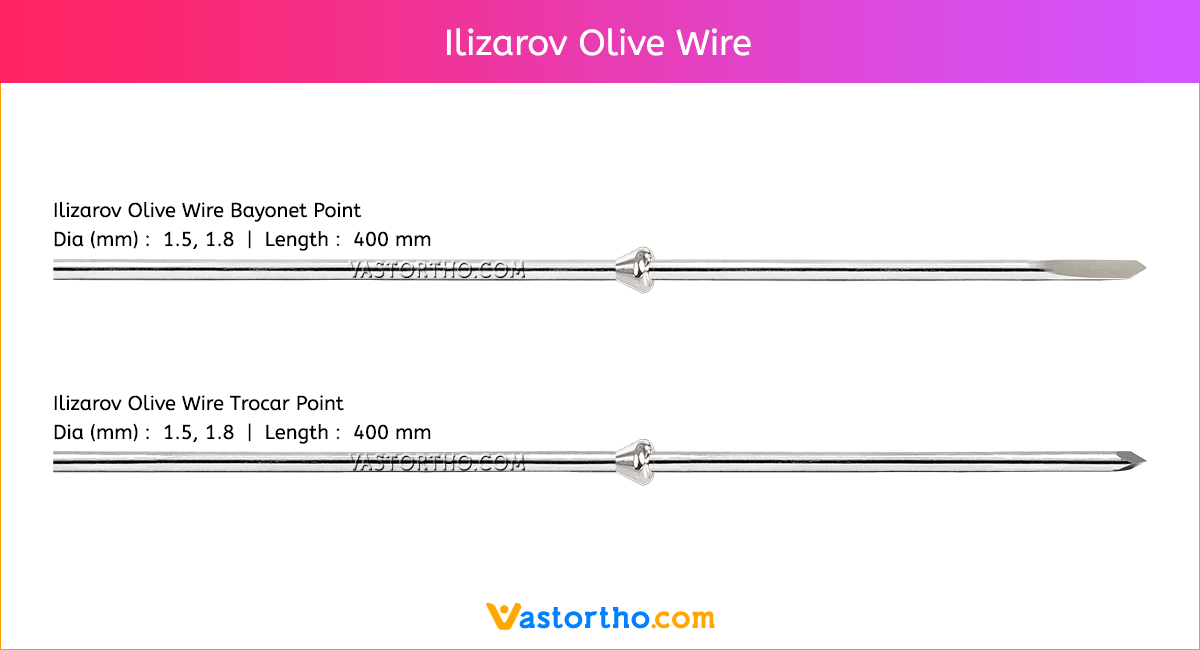
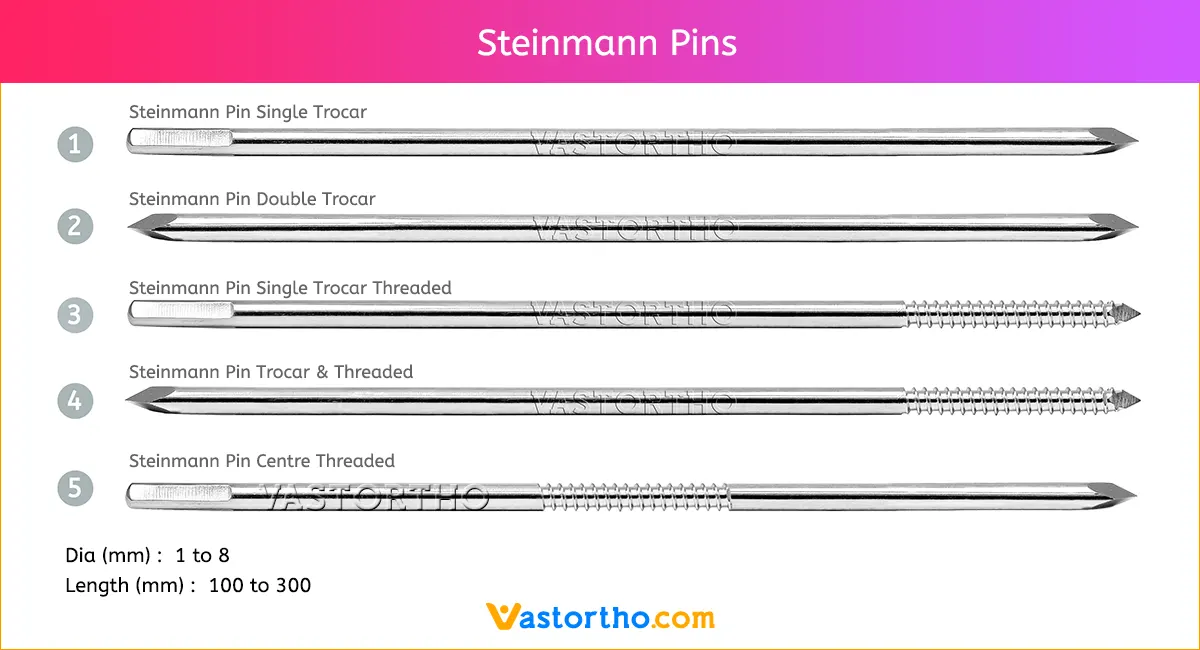
Steinmann Pins are available in various Types, lengths and diameters.
Types: Single Trocar, Double Trocar, Single Trocar Threaded, Trocar & Threaded, Centre Threaded
Diameters: 1mm, 2mm, 2.5mm, 3mm, 3.5mm, 4mm, 4.5mm, 5mm, 5.5mm, 6mm, 6.5mm, 7mm and 8mm
Lengths: 100 mm to 300 mm