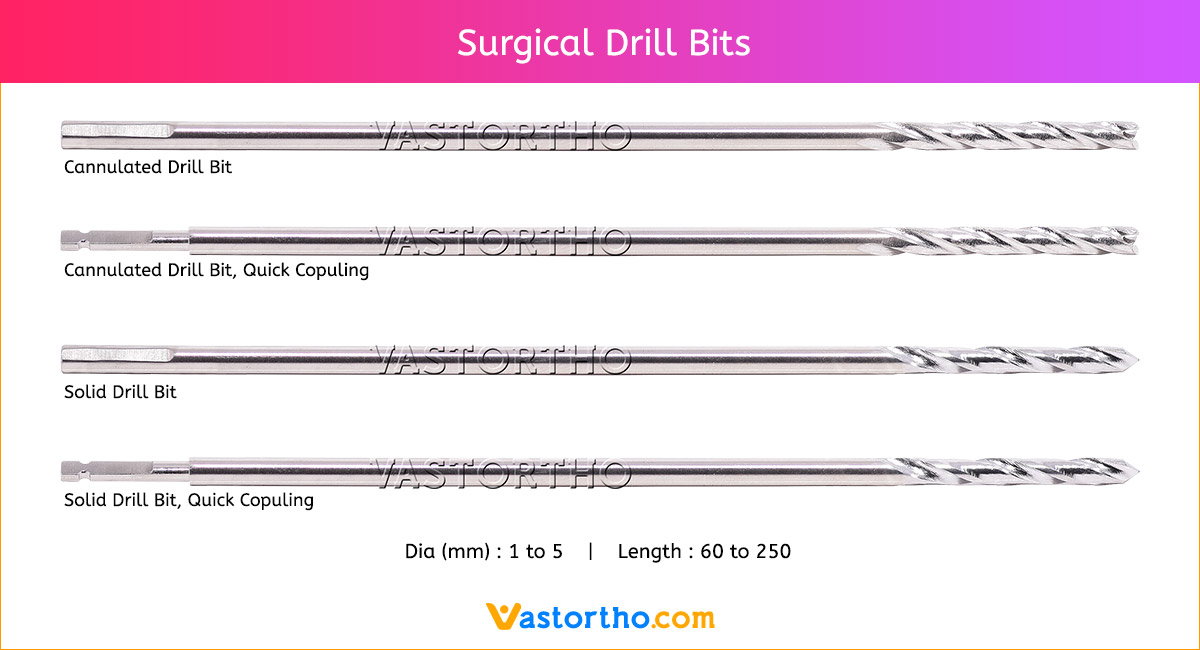
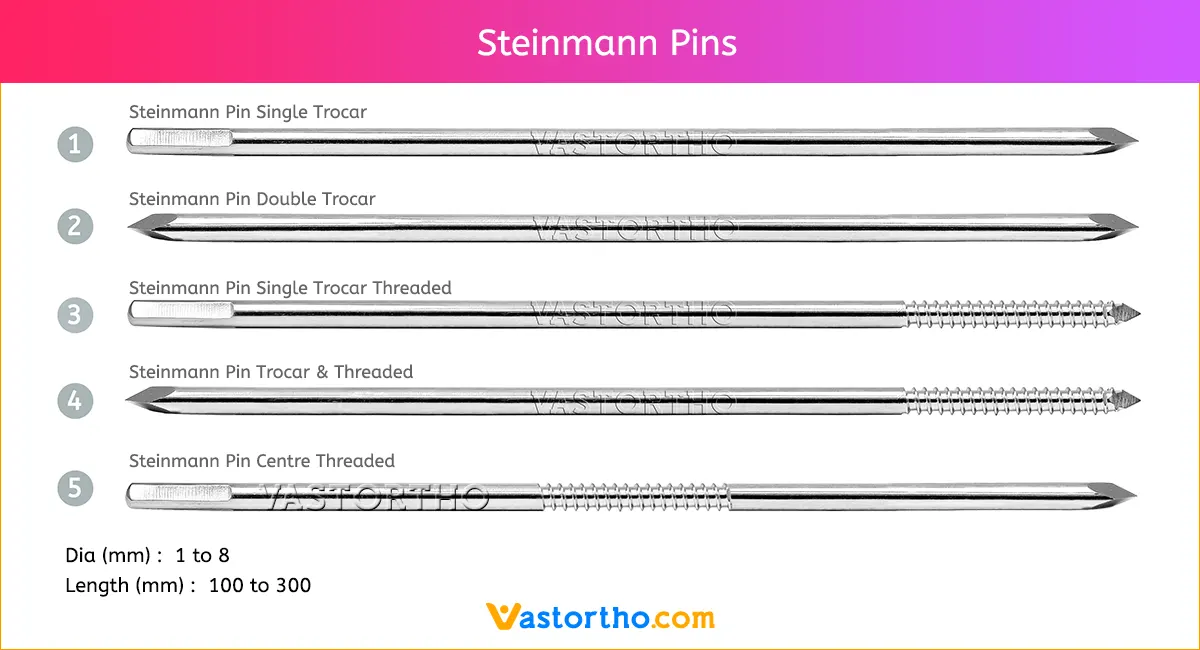
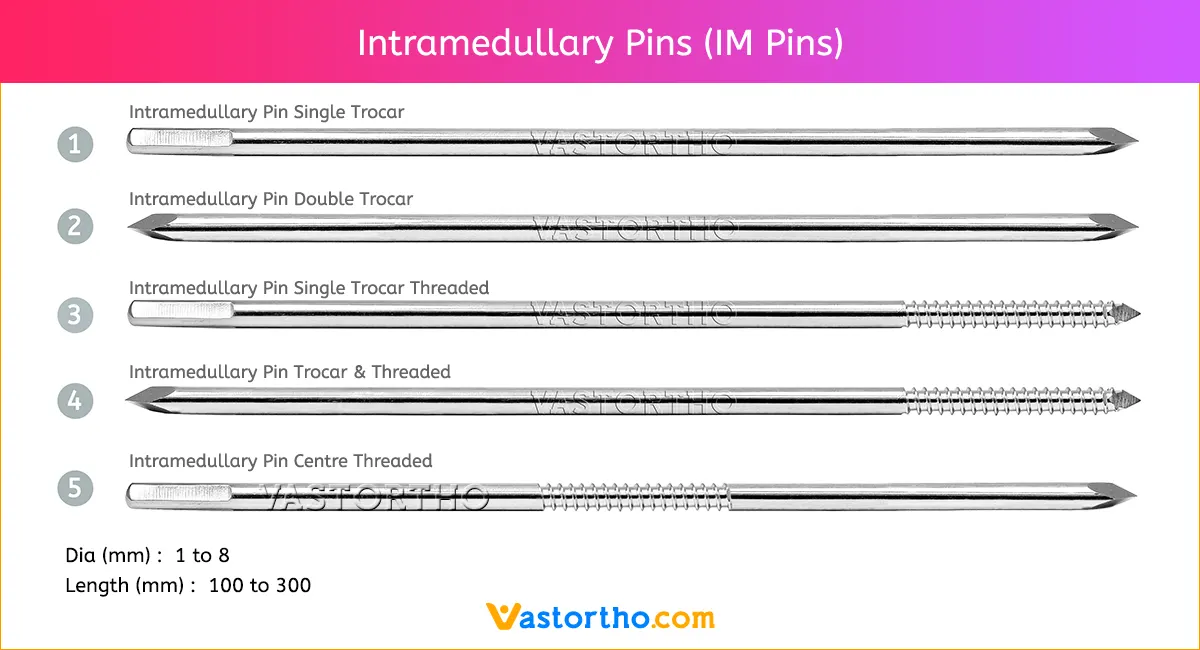
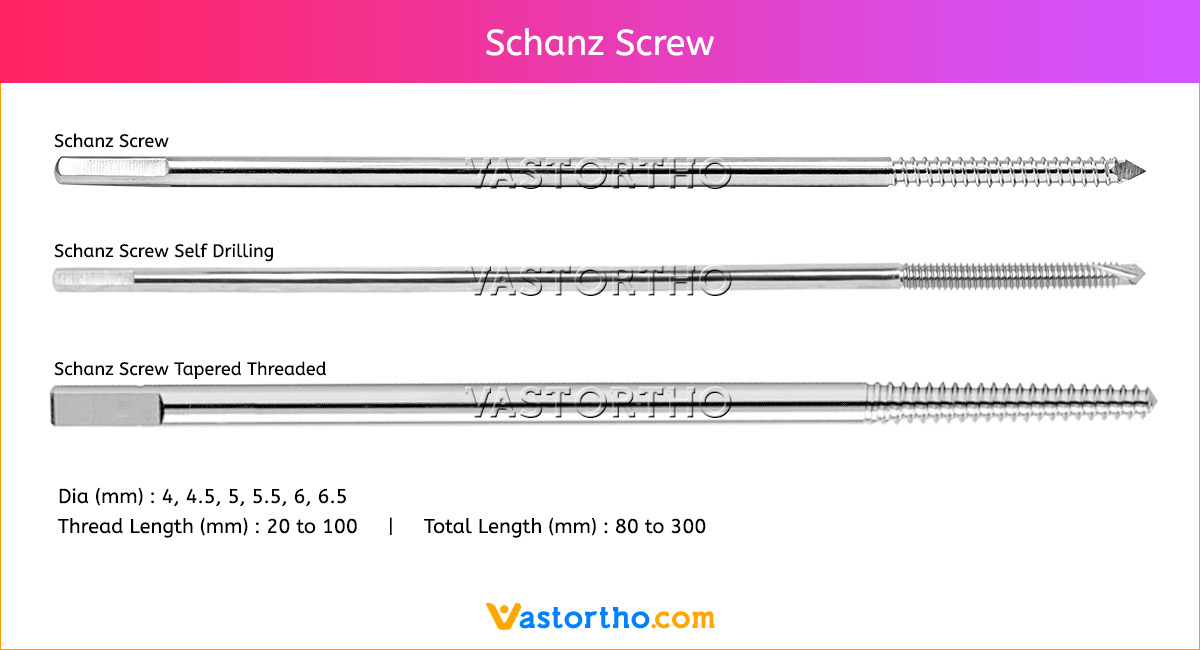
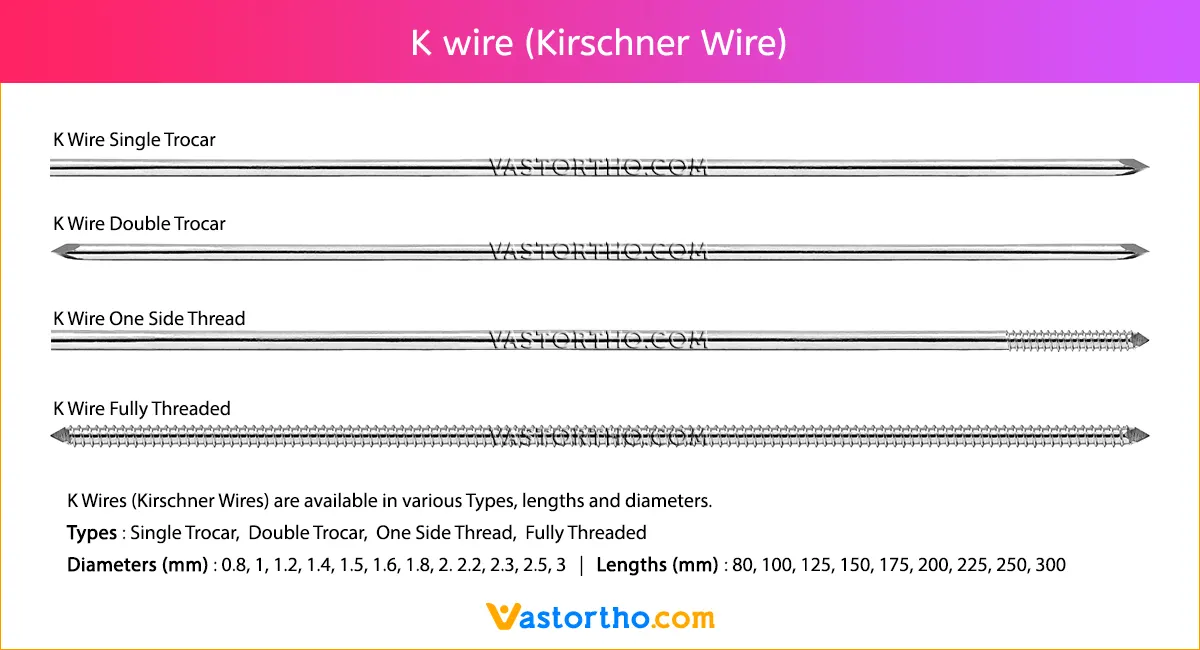
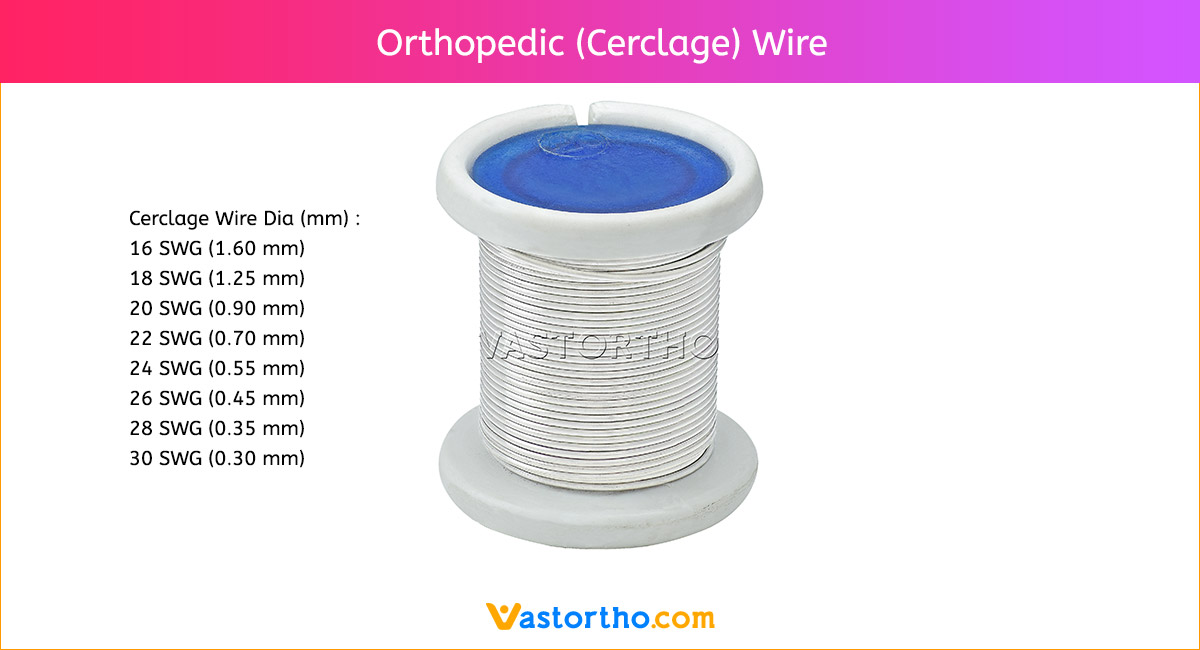
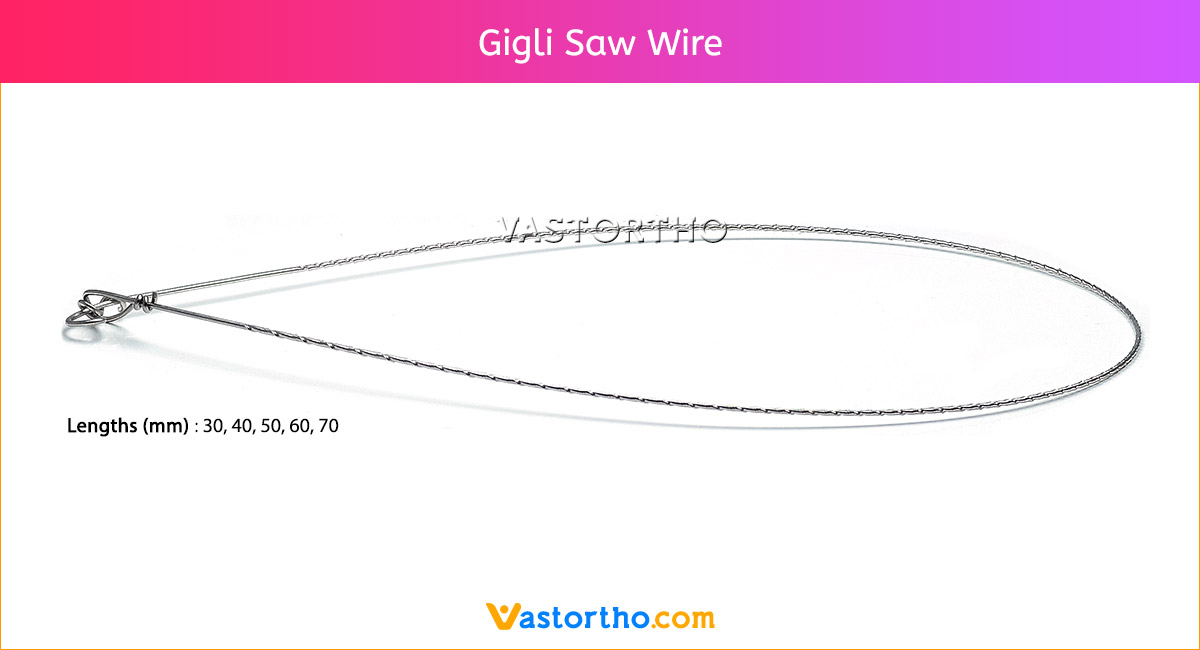
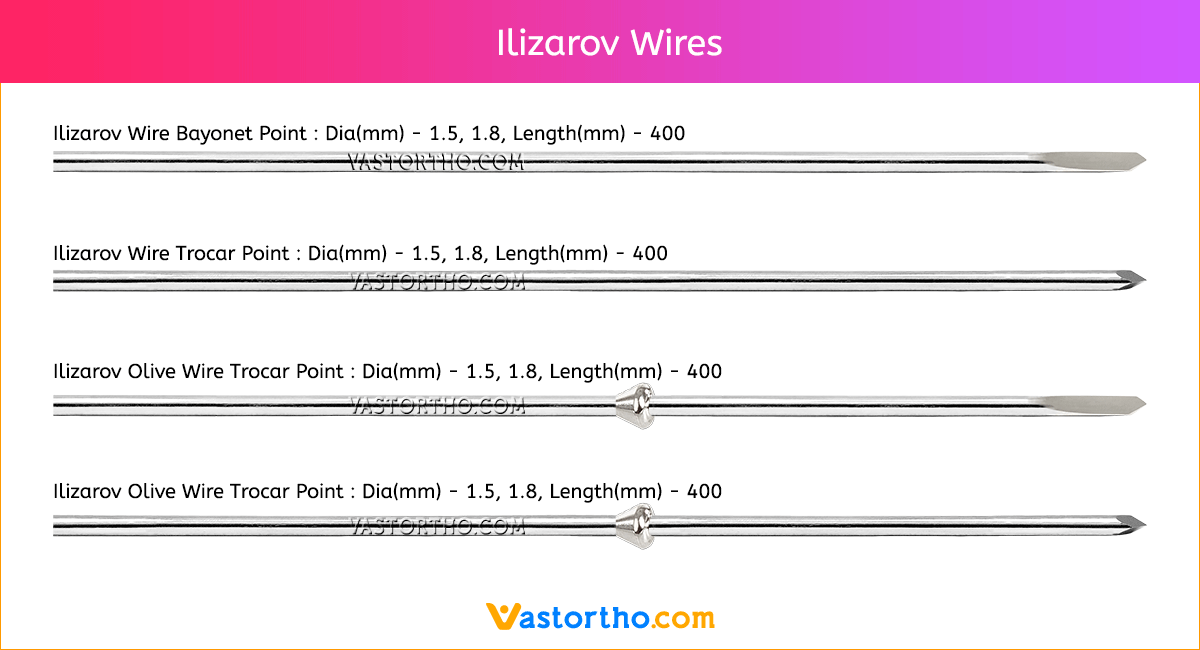
Guide Wire PFN Specification, Uses, Sizes and Surgical Techniques.
Most nails are inserted over a guide wire. Reaming is always performed over a guide wire.
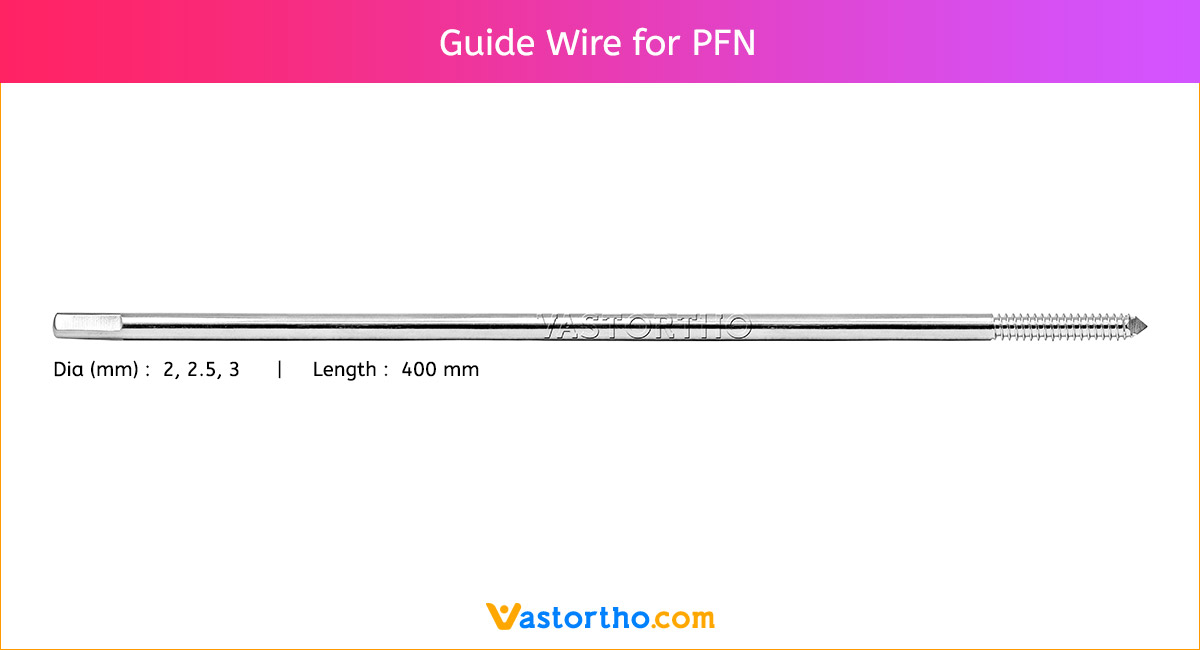
The Guide Wire PFN is inserted under X-ray control.
In order to facilitate passing the guide wire across the fracture site, the fracture should be adequately aligned. It may be easier to pass the guide wire if the tip is angulated.
If the fracture ends cannot be sufficiently aligned for a guide wire to passed, an aiming device may be inserted into the proximal fragment (after reaming if necessary) to manipulate the proximal fragment so that the guide wire PFN can be passed into the distal fragment.
The correct length of the nail is determined by comparing a second guide wire of the same length to the one that has been inserted. The correct placement of the guide wire PFN in the distal canal should be assessed via image intensifier. The tip of the second guide wire must be positioned at the entry point in the bone.