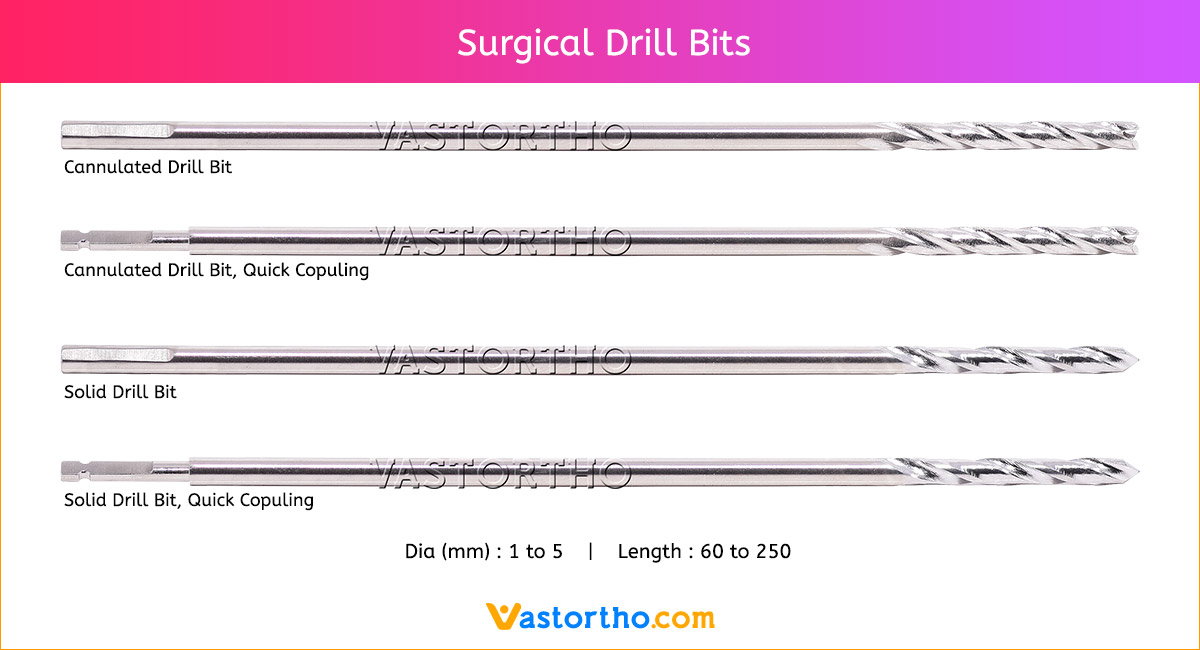
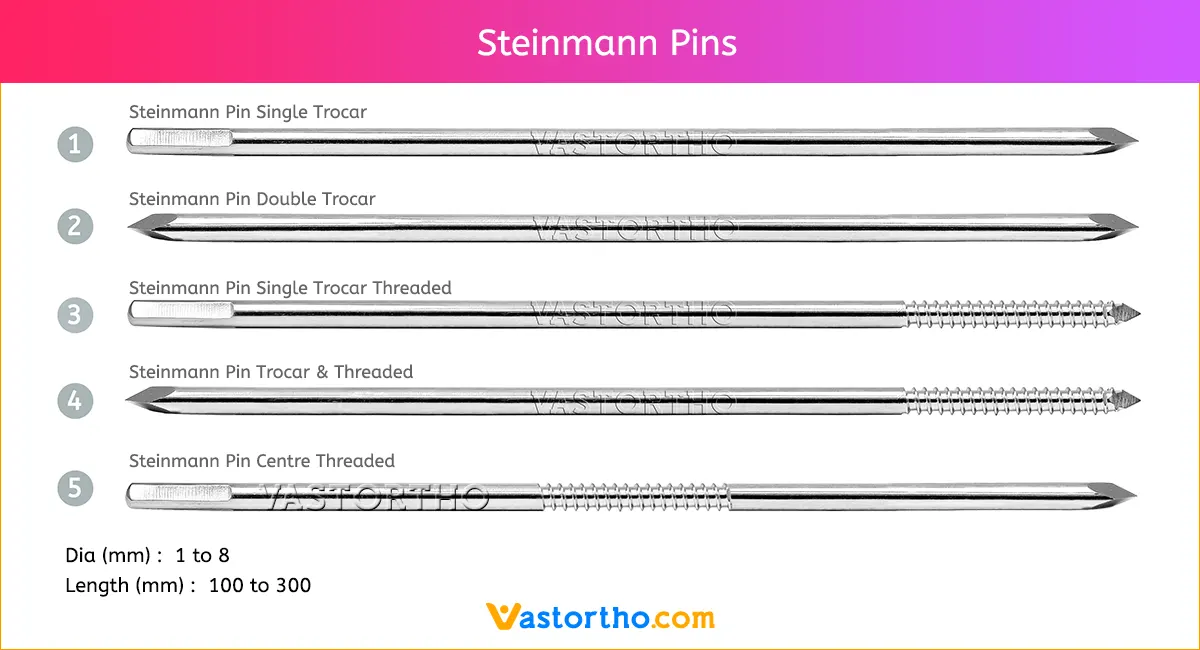
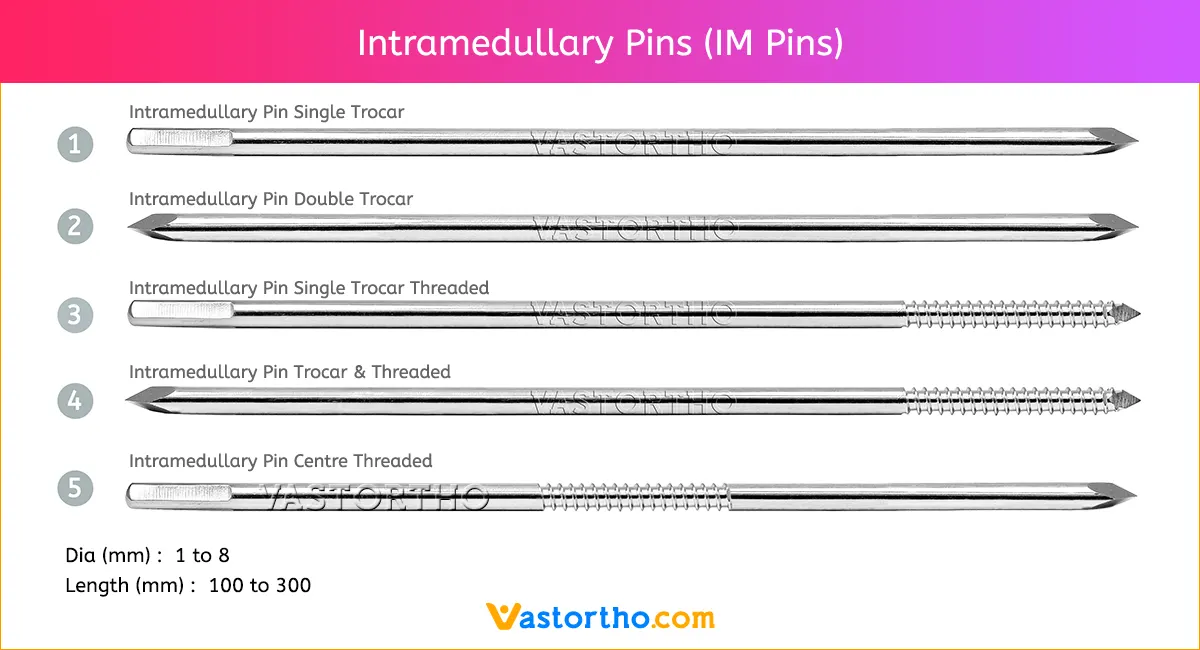
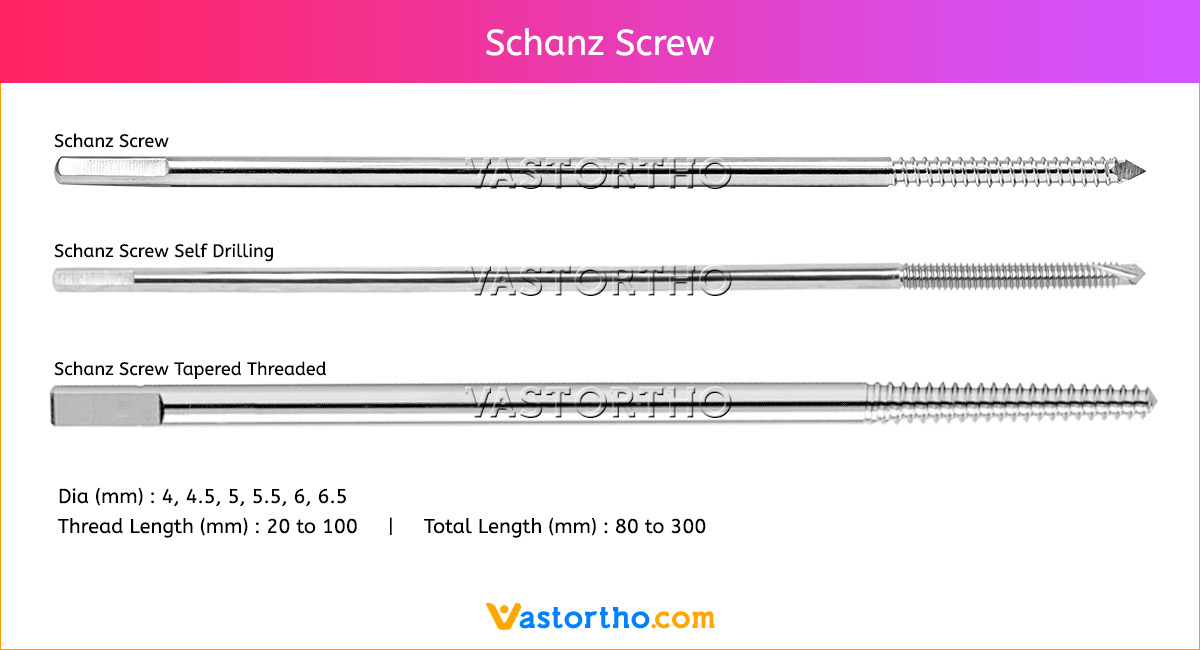
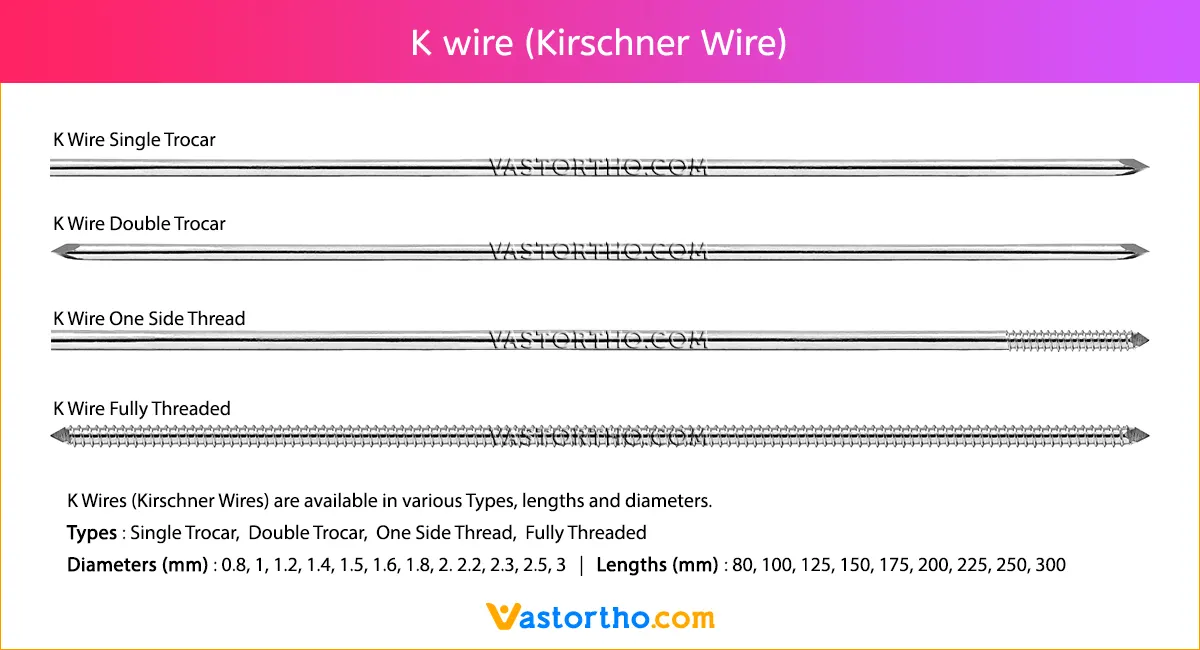
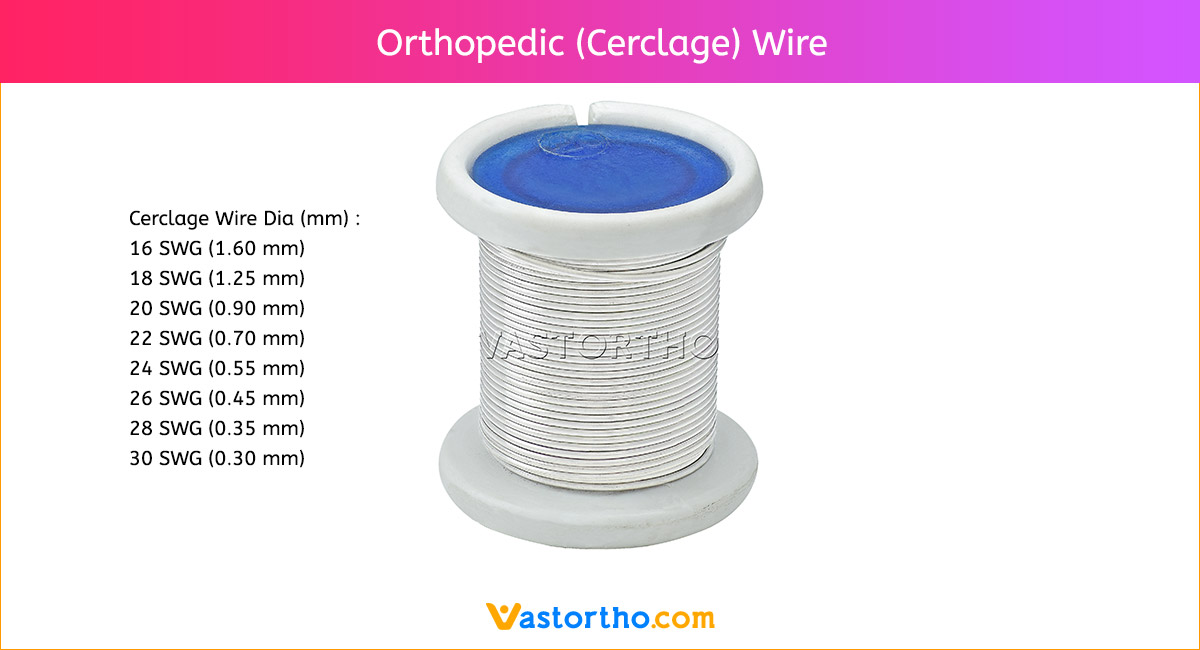
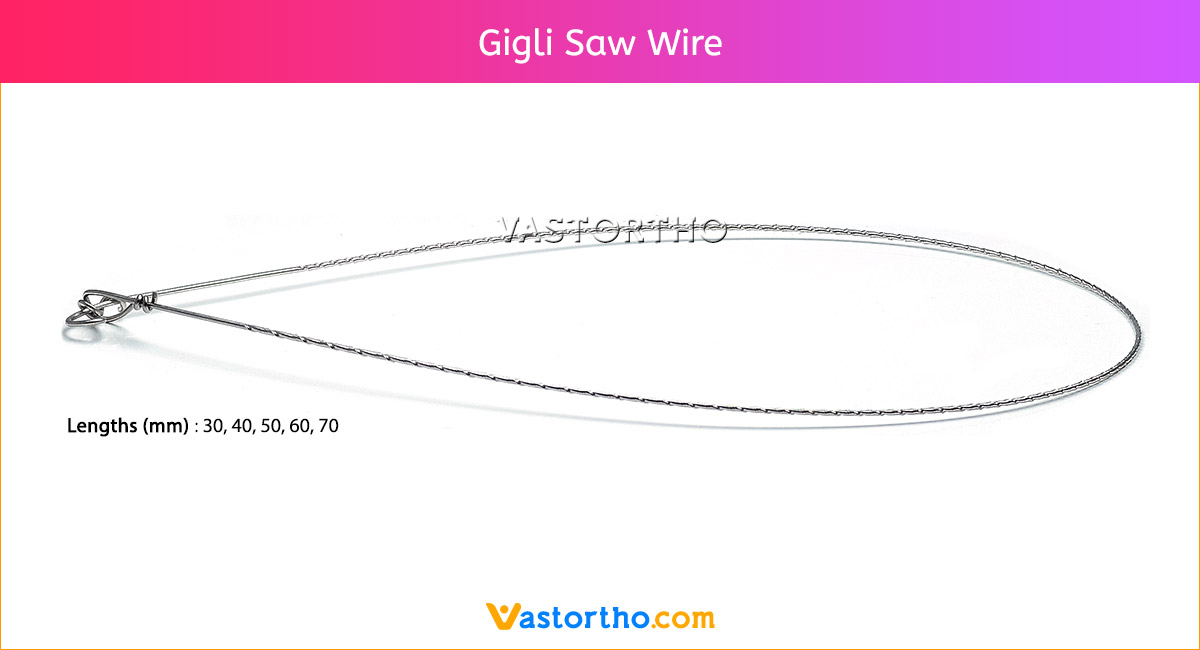
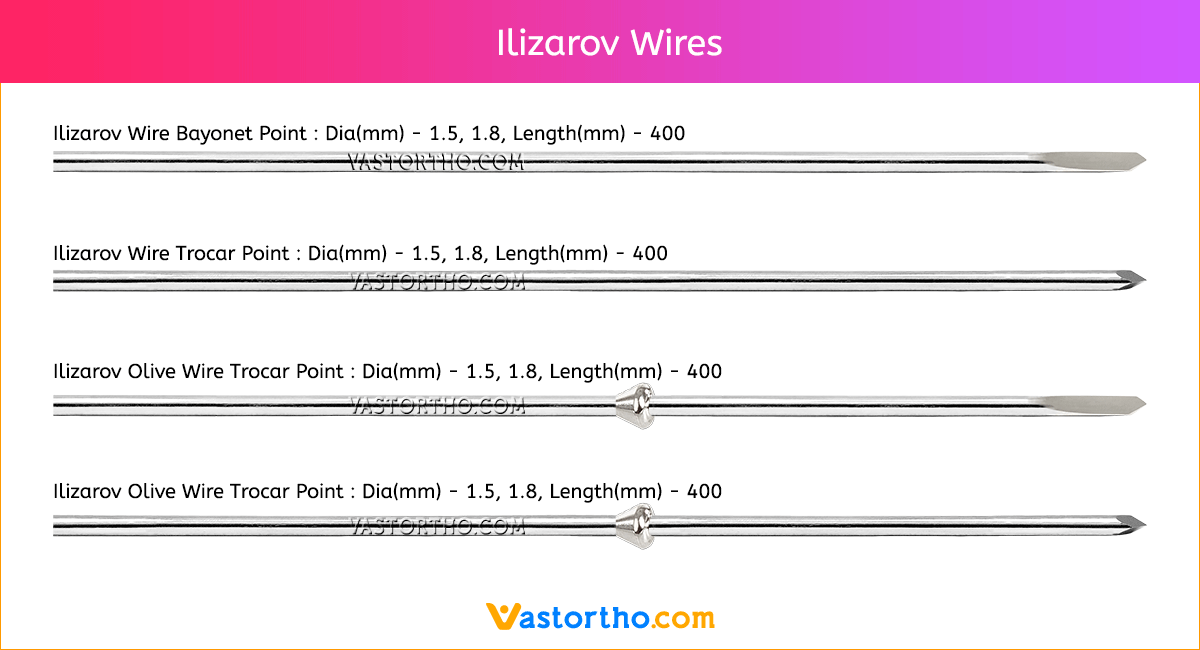
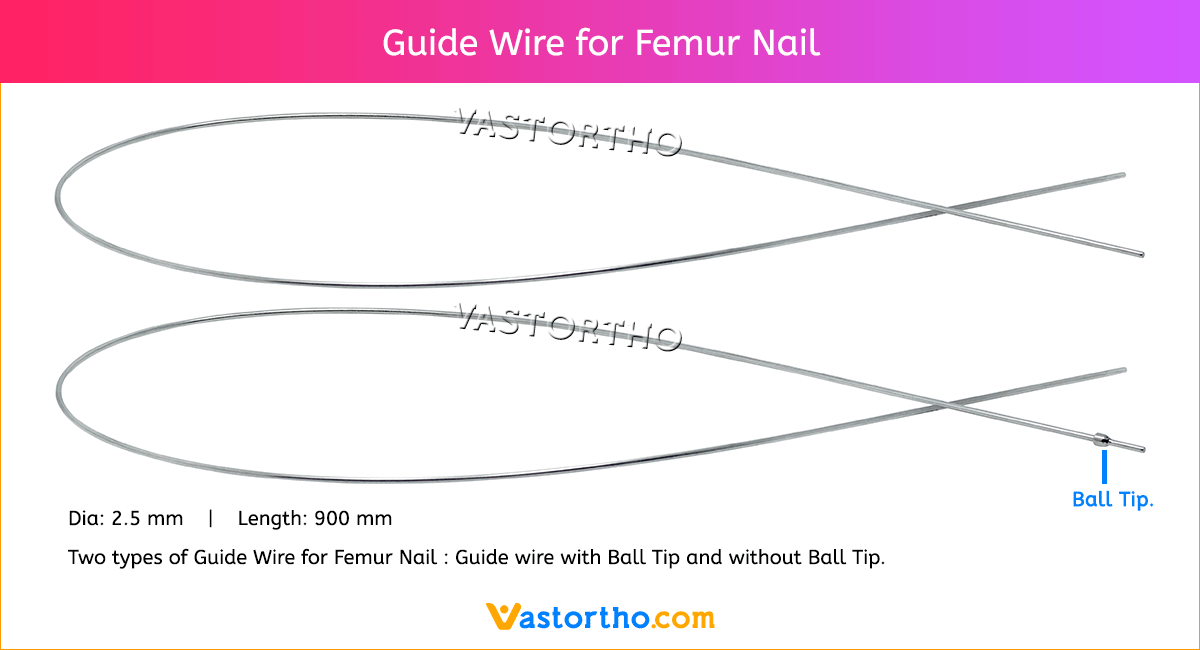
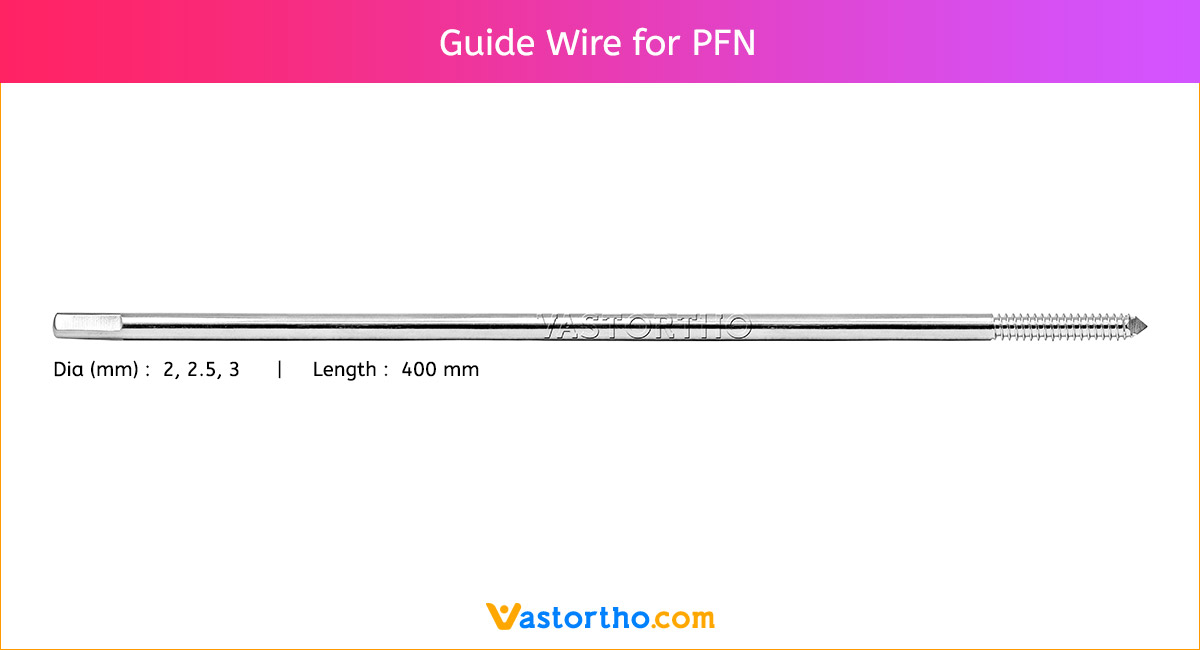
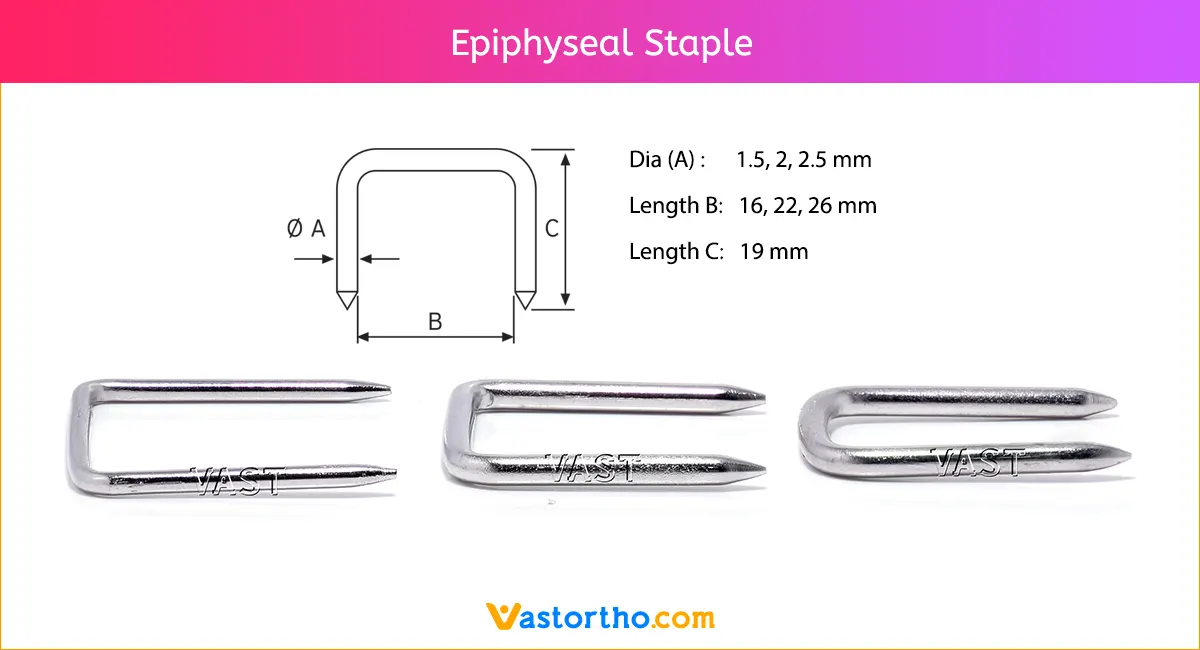
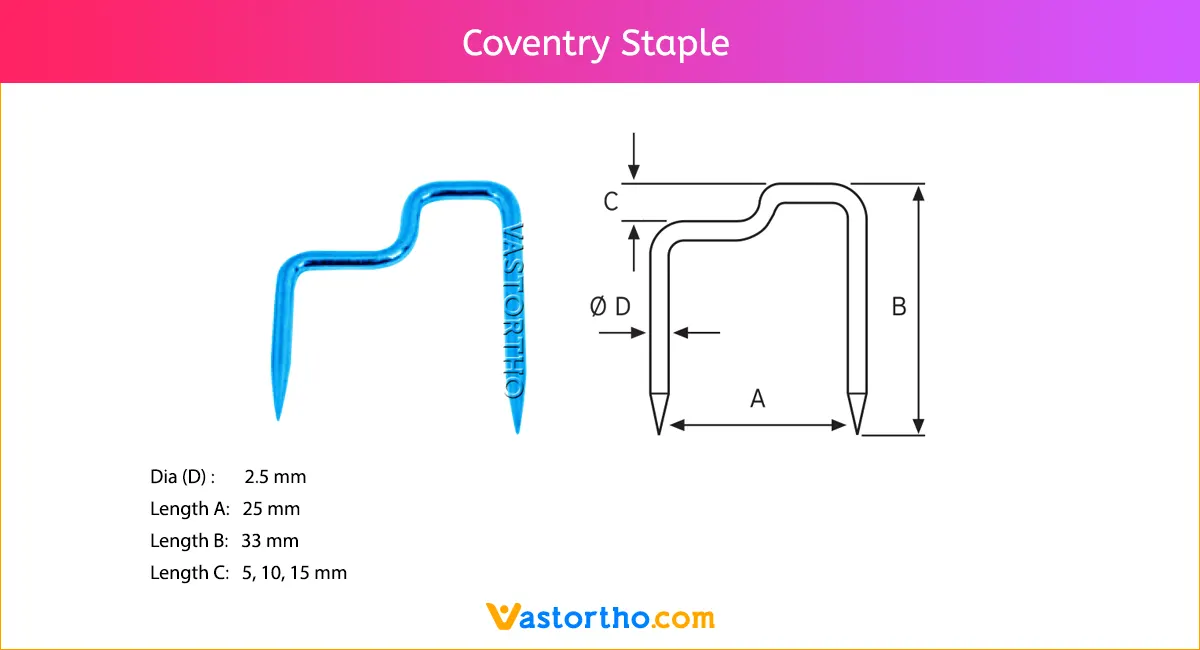
Ilizarov Olive Wire Specification, Uses, Sizes and Surgical Techniques.
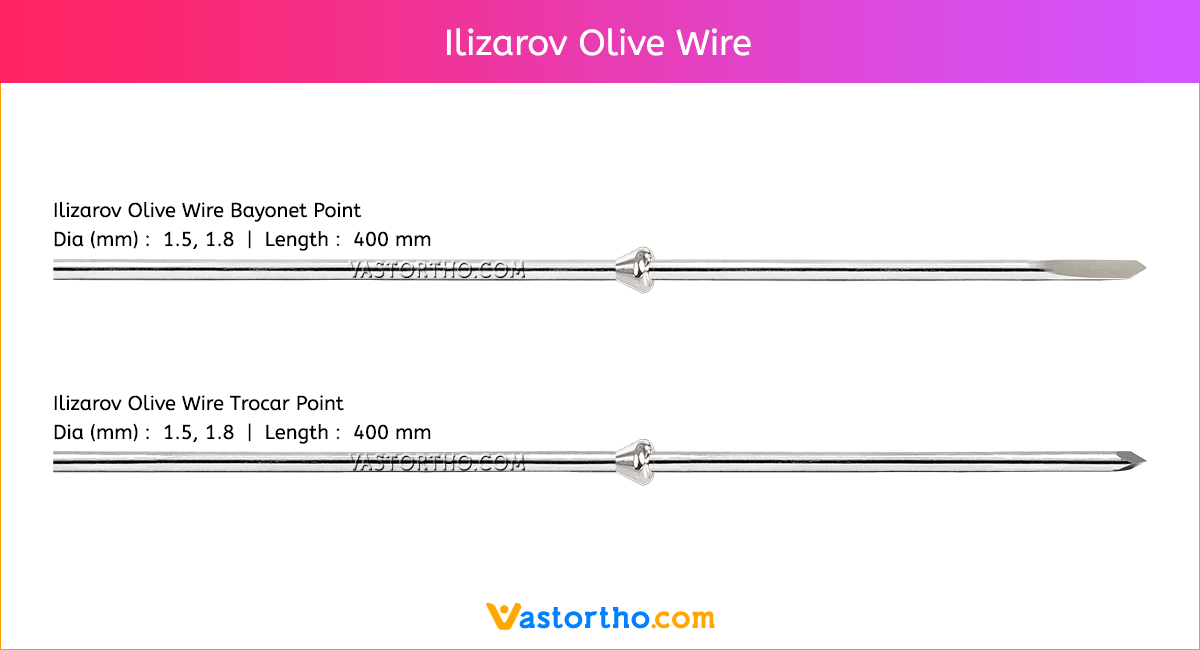
Ilizarov Olive Wire fixators are used to treat fractures that are comminuted or near articular surfaces. They are also widely used in the corrective treatment of non-unions, post-trauma residual misalignment, and limb deformities (by distraction osteogenesis).The Ilizarov method of fracture fixation makes use of pre-tensioned, thin Ilizarov Olive Wire that transfix the bone, supported by circular rings, which are connected using stiff longitudinal bars. Unlike other fixation methods, Ilizarov fixators are characterized by non-linear stiffness in the axial direction. The pre-tensioned Ilizarov Olive Wire behave like beams and cables simultaneously, but with increasing load the cable behavior dominates, and their load carrying capacity changes non-linearly with the sagging of the wires. This results in a geometrically non-linear response in the form of a non-linear load-deflection curve.