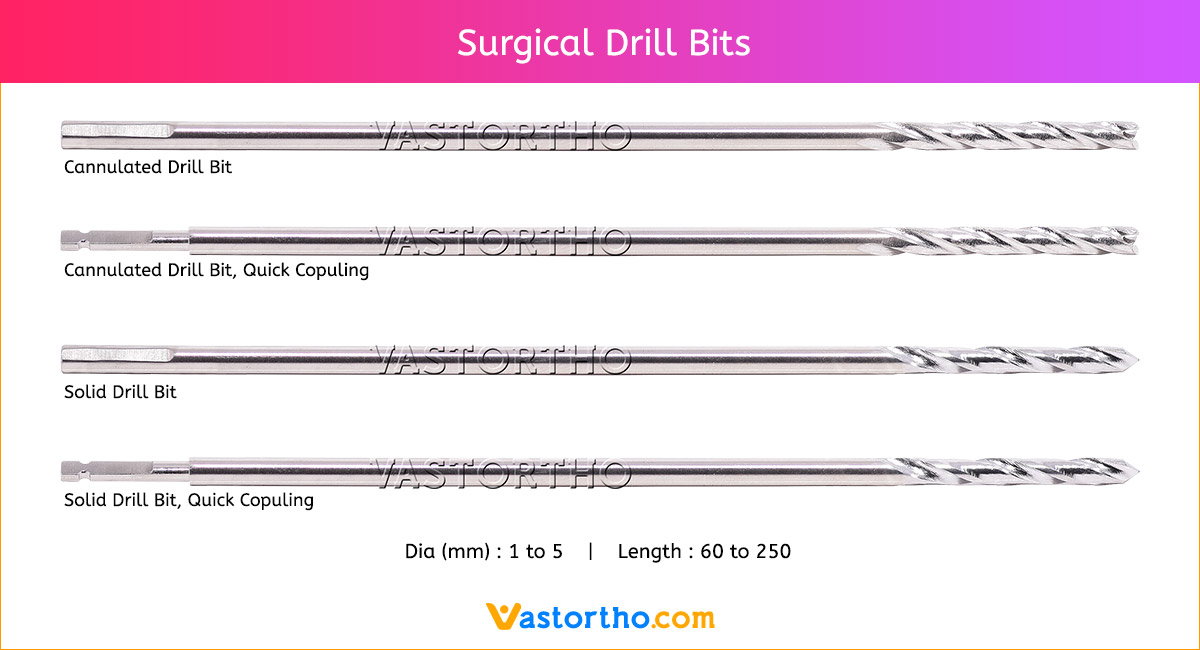
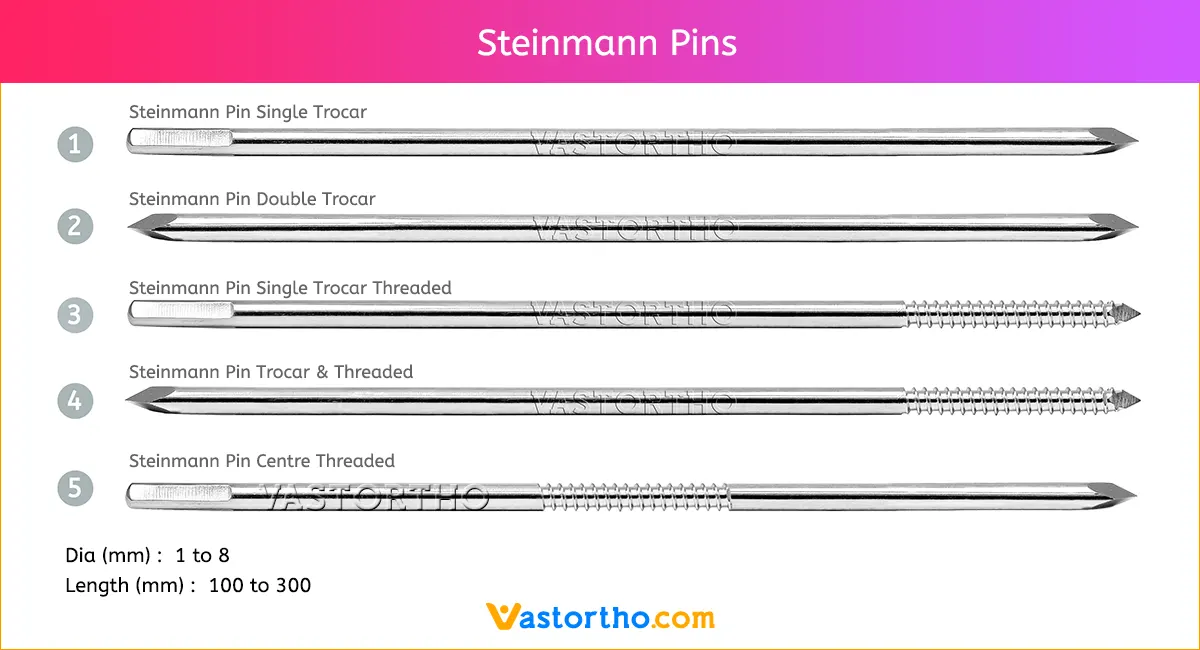
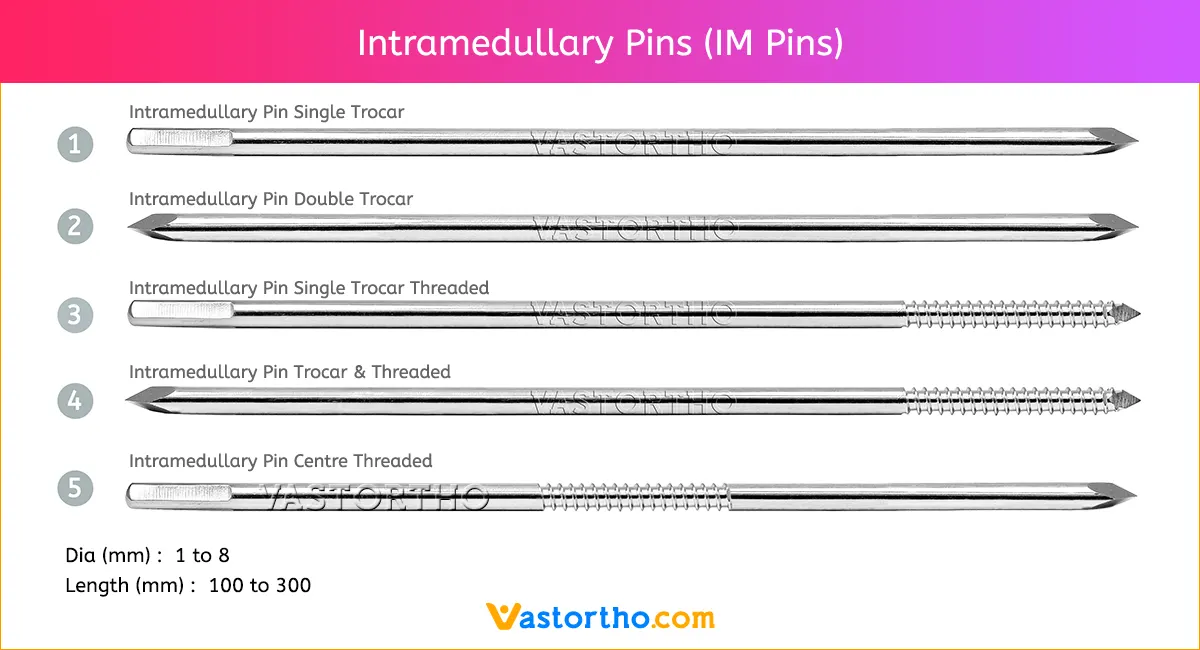
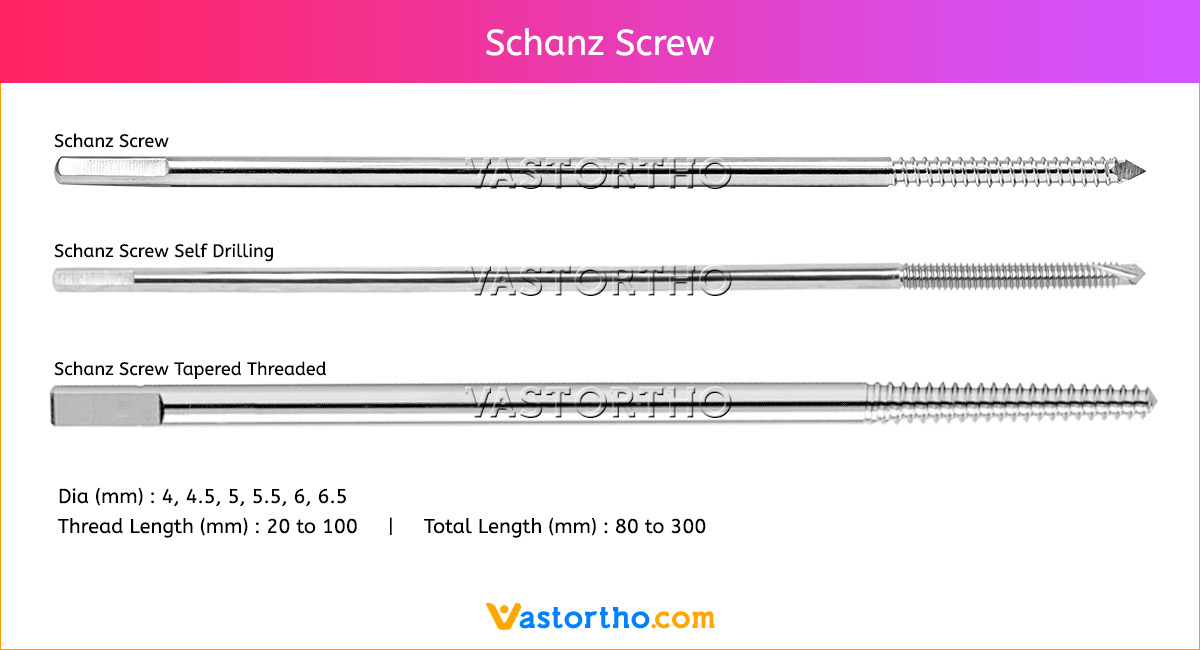
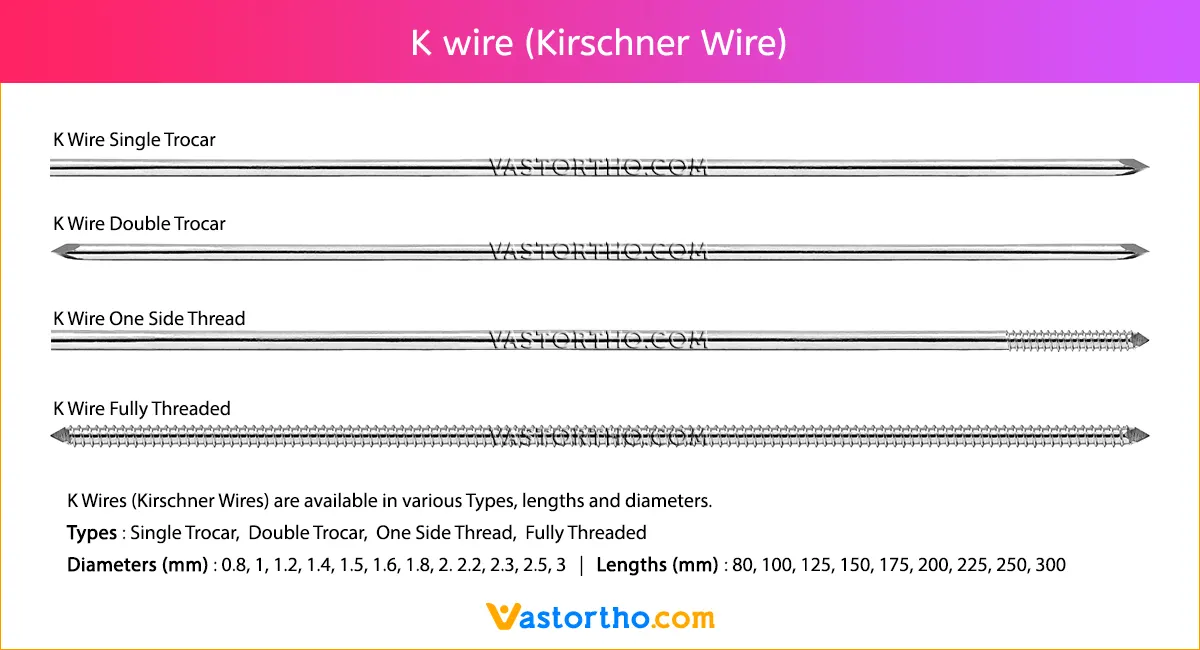
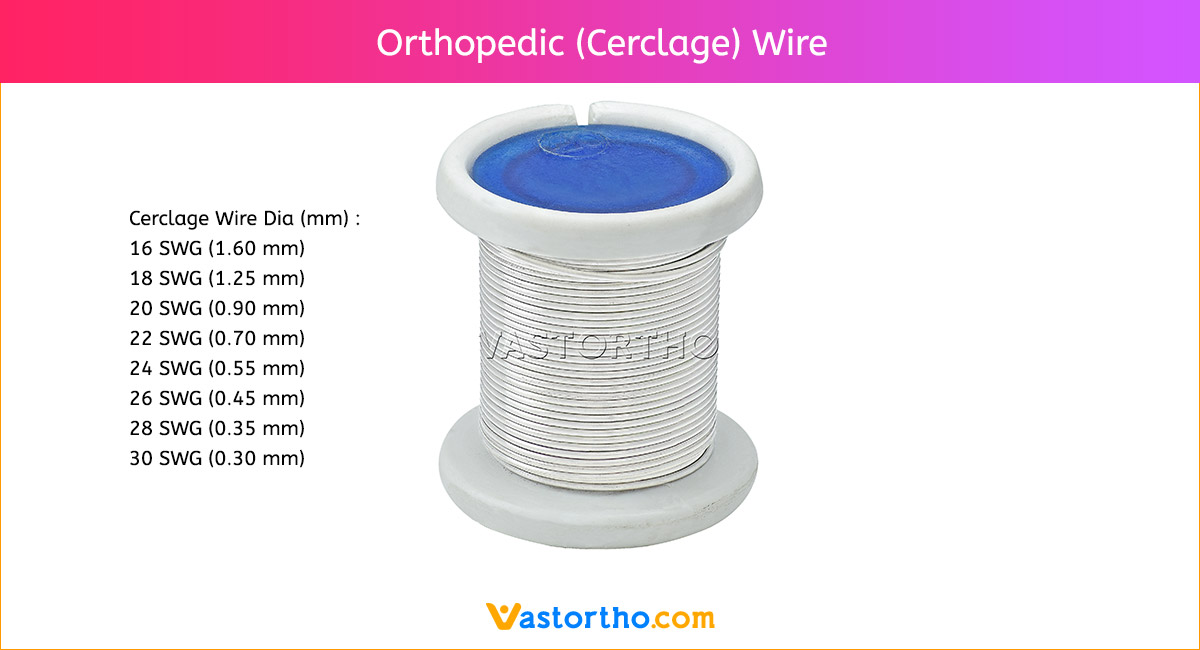
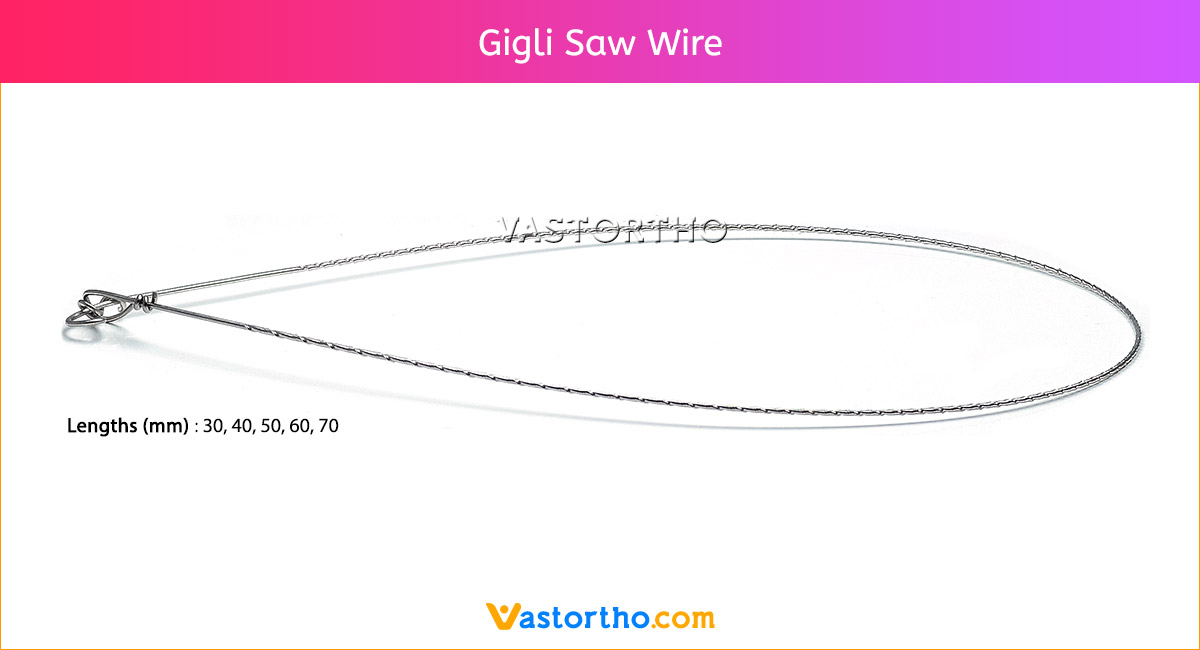
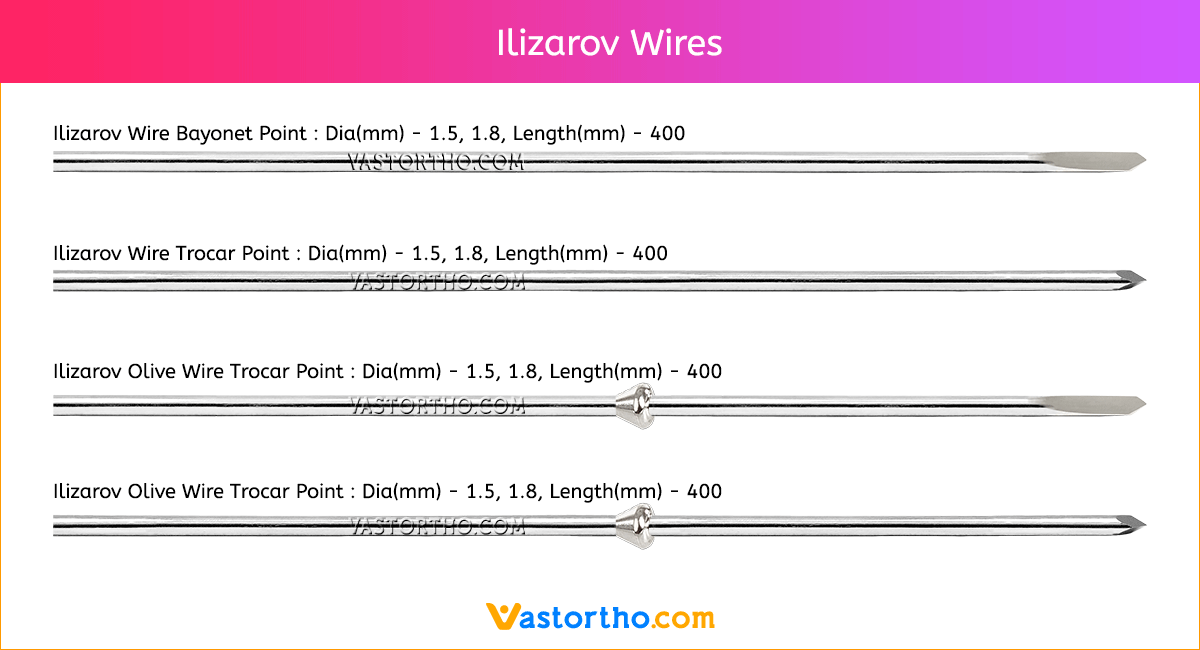
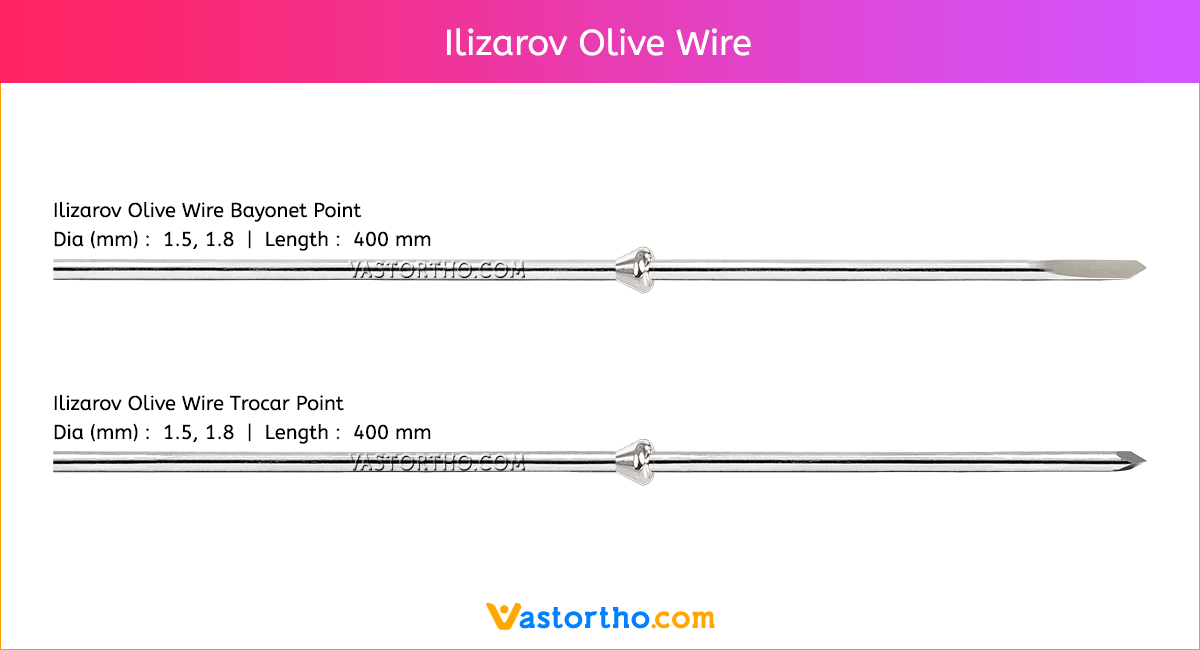
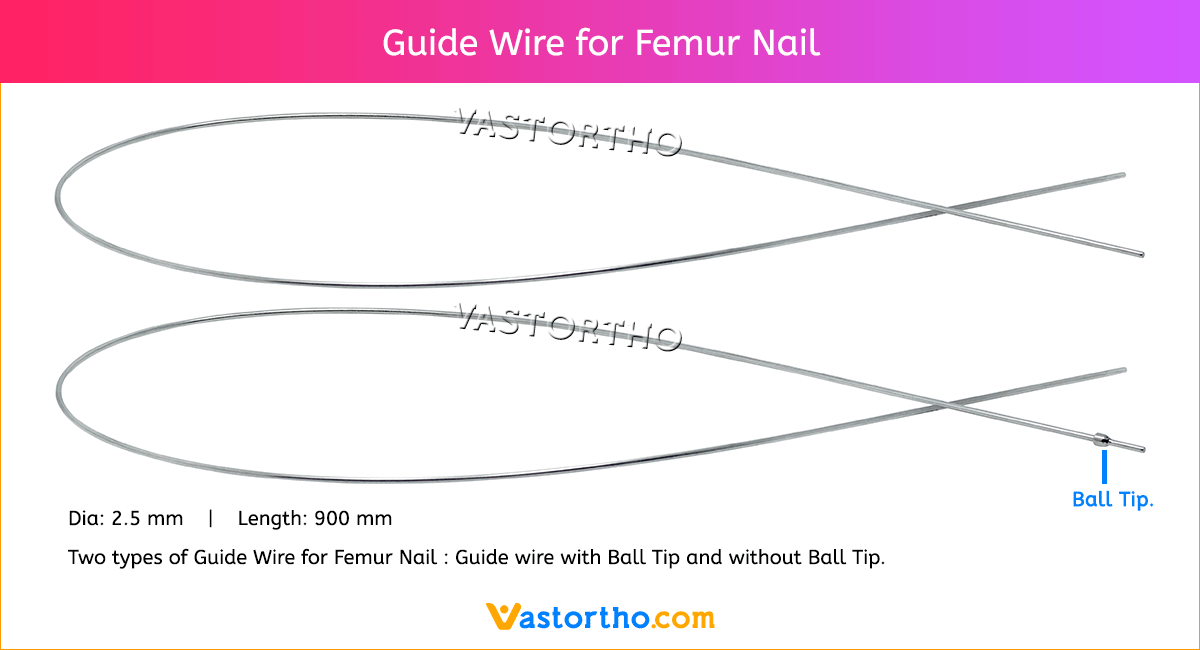
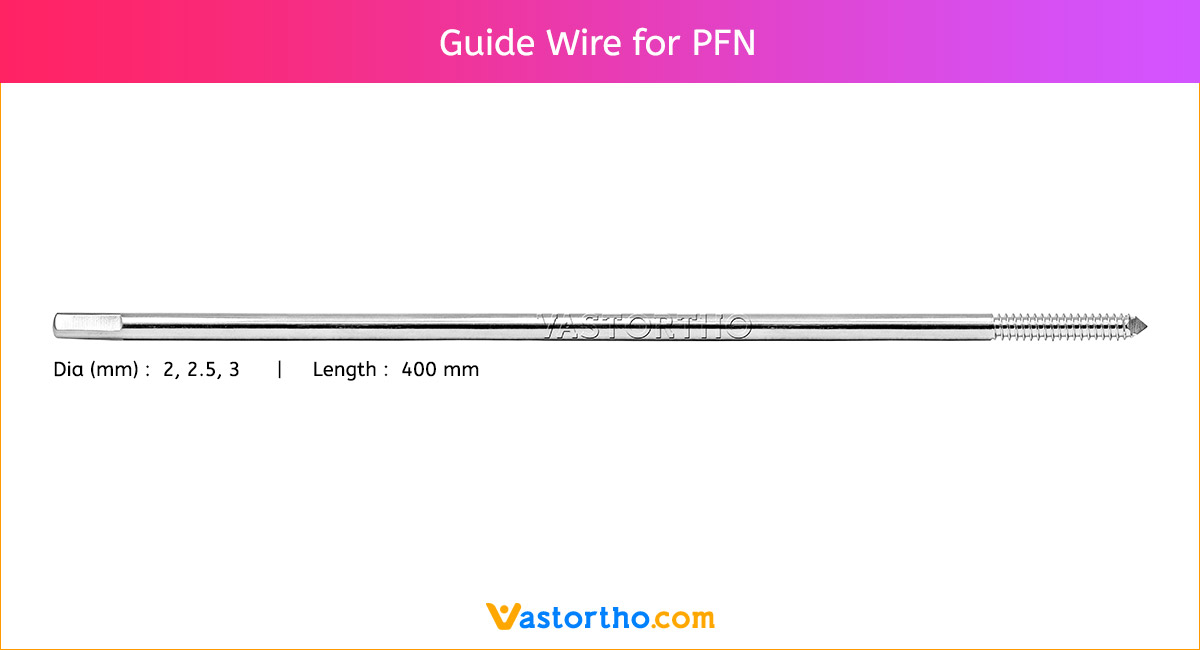
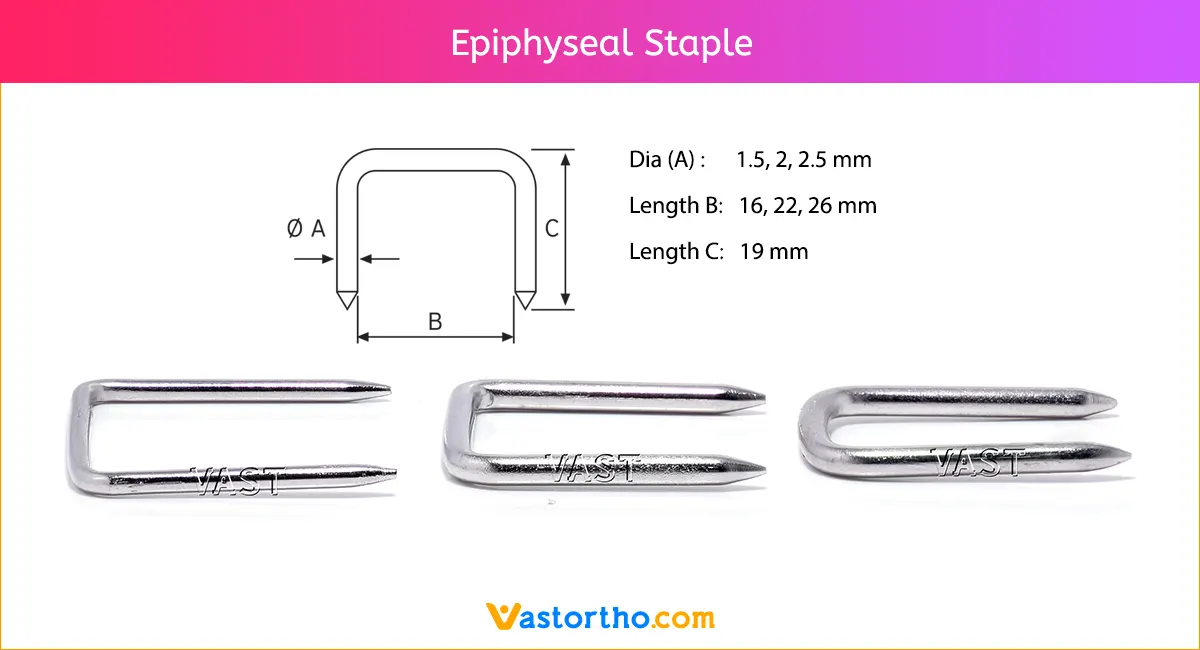
Guide Wire Cannulated Screw Specification, Uses, Sizes and Surgical Techniques.

A Guide Wire Cannulated Screw is a thin, flexible wire inserted into the body to guide cannulated screws during surgery. Our Guide Wire is made from finest quality of SS 316L material to ensure highest quality. Cannulated Screw Guide Wire Specifications are:
- 1.2 mm Diameter and 230 mm Length for 4 mm Cannulated Screw
- 2 mm Diameter and 230 mm Length for 6.5 mm Cannulated Screw
The main advantage of cannulated screws is that they can be inserted over a guide wire. The diameter of the Guide Wire Cannulated Screw is much smaller than the cannulated screw and can be more accurately placed using fluoroscopy in the operating room. In addition, given its small diameter, the Guide Wire can be reinserted several times if necessary for accurate placement without excessive damage to bone.