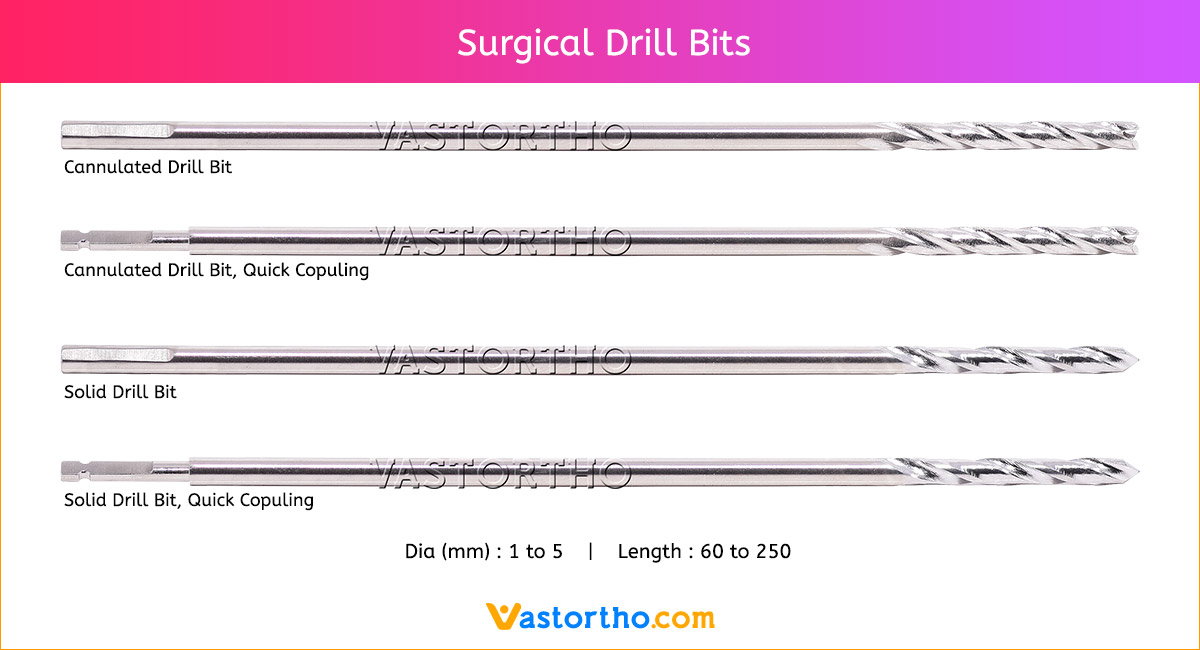
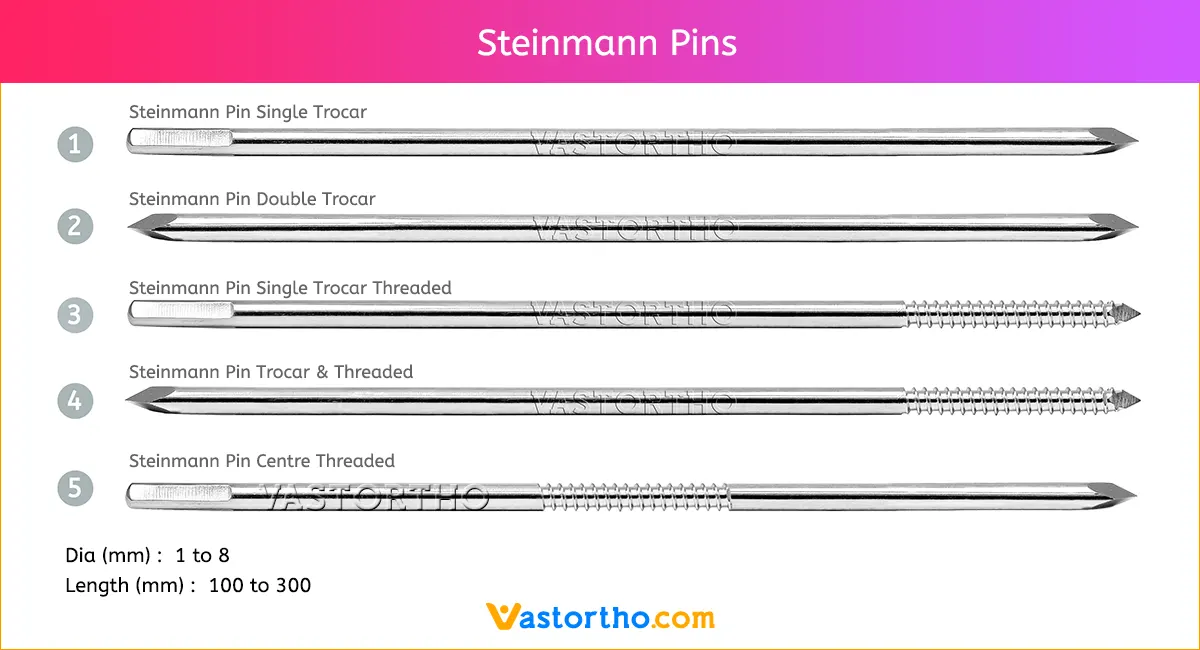
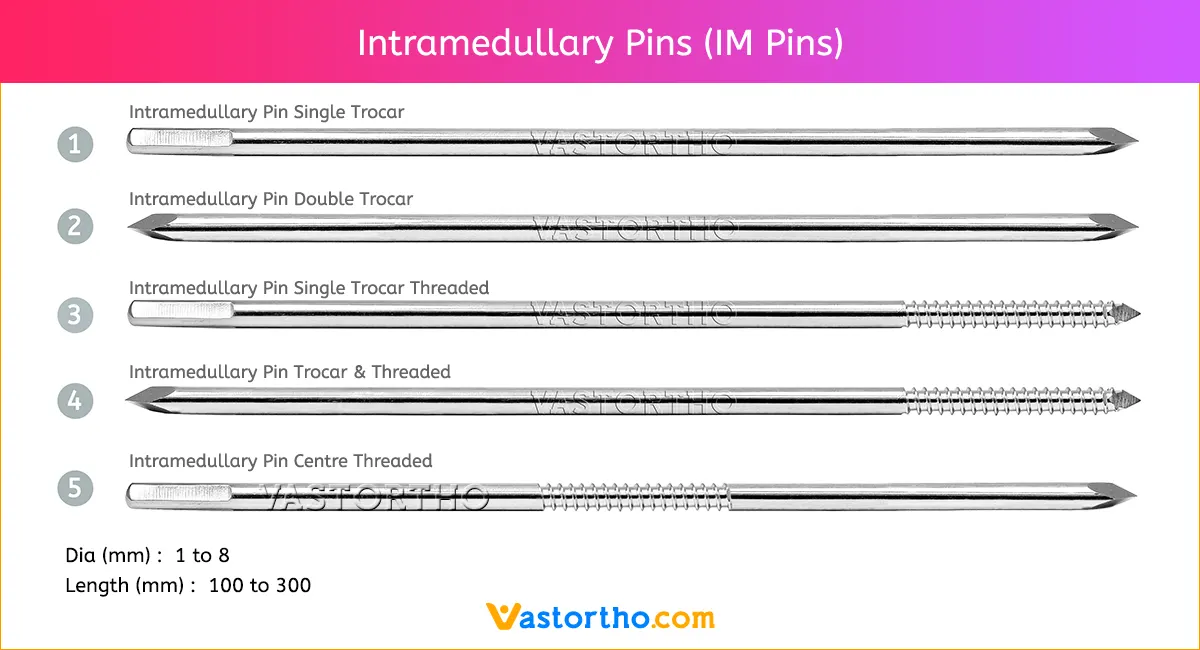
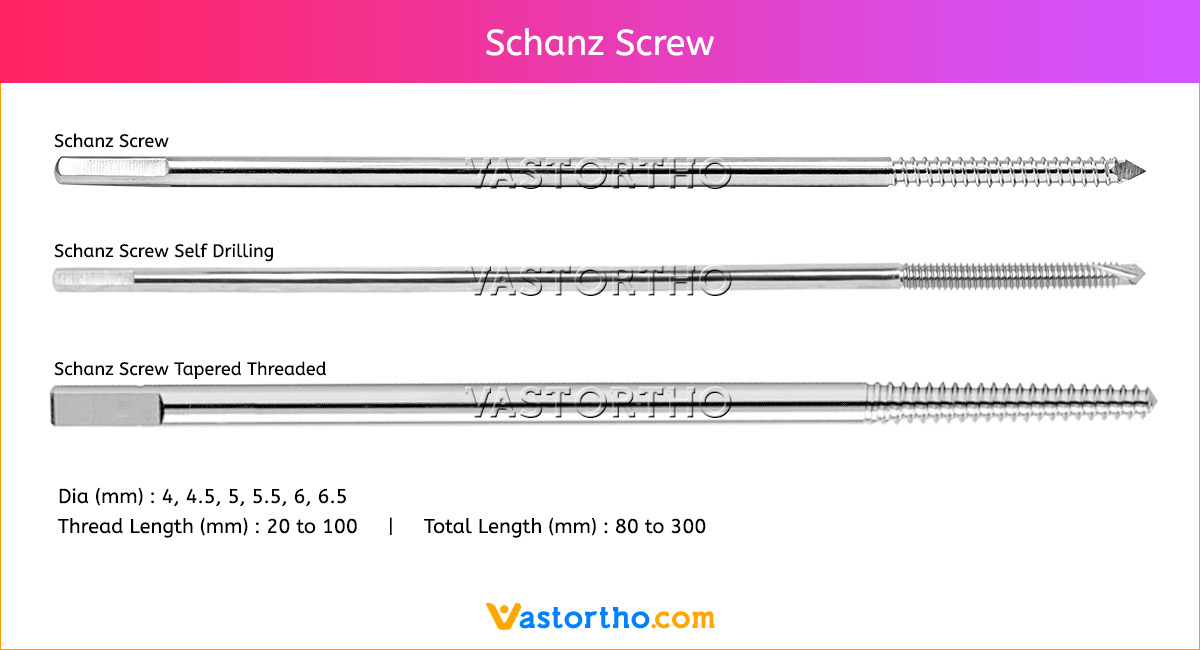
Epiphyseal Staple Specification, Uses, Sizes and Surgical Techniques.
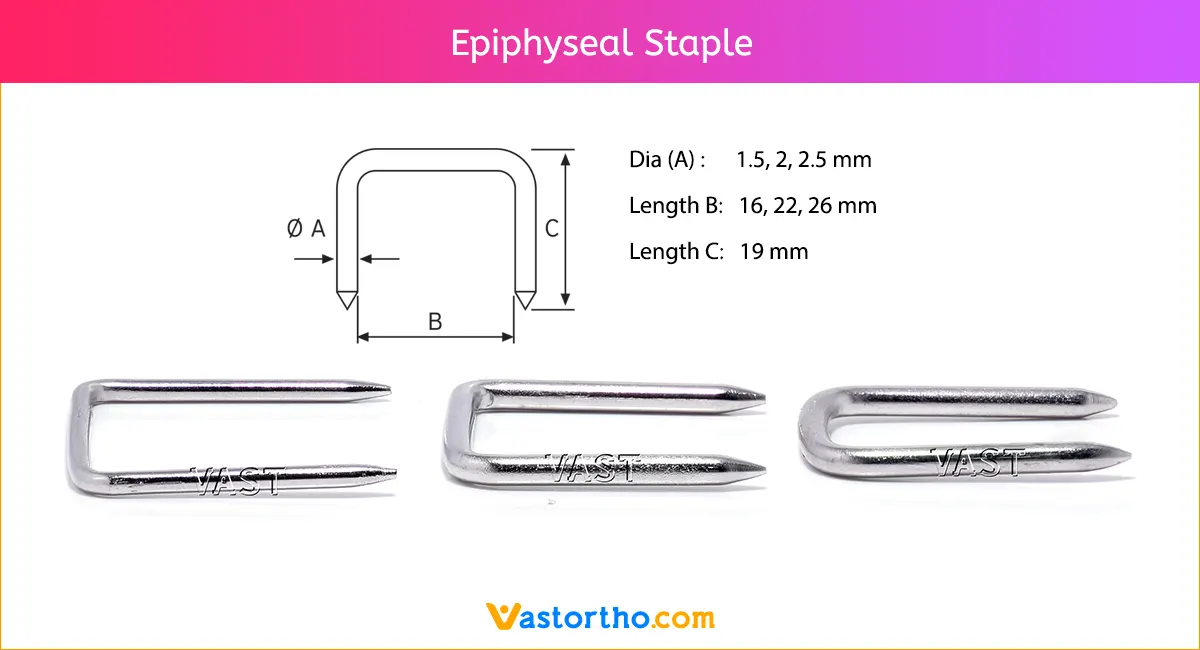
Epiphyseal Staple is used for correcting angular deformity at the knee. Two or more Epiphyseal Staple should be used on each side of the epiphysis.
Our Epiphyseal Staples are made from finest quality medical grade material to ensure highest quality. Different sizes of Epiphyseal Staples are:
- Dia(A) x Length(B) x Length(C) = 1.5 mm x 16 mm x 19 mm
- Dia(A) x Length(B) x Length(C) = 1.5 mm x 22 mm x 19 mm
- Dia(A) x Length(B) x Length(C) = 1.5 mm x 26 mm x 19 mm
- Dia(A) x Length(B) x Length(C) = 2.0 mm x 16 mm x 19 mm
- Dia(A) x Length(B) x Length(C) = 2.0 mm x 22 mm x 19 mm
- Dia(A) x Length(B) x Length(C) = 2.0 mm x 26 mm x 19 mm
- Dia(A) x Length(B) x Length(C) = 2.5 mm x 16 mm x 19 mm
- Dia(A) x Length(B) x Length(C) = 2.5 mm x 22 mm x 19 mm
- Dia(A) x Length(B) x Length(C) = 2.5 mm x 26 mm x 19 mm