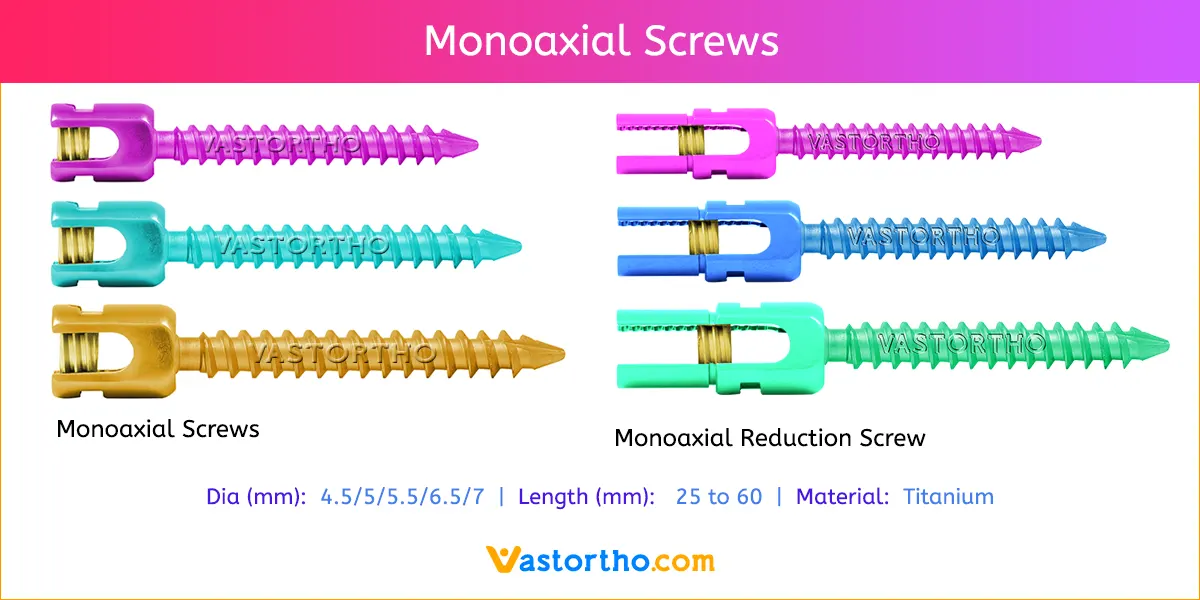
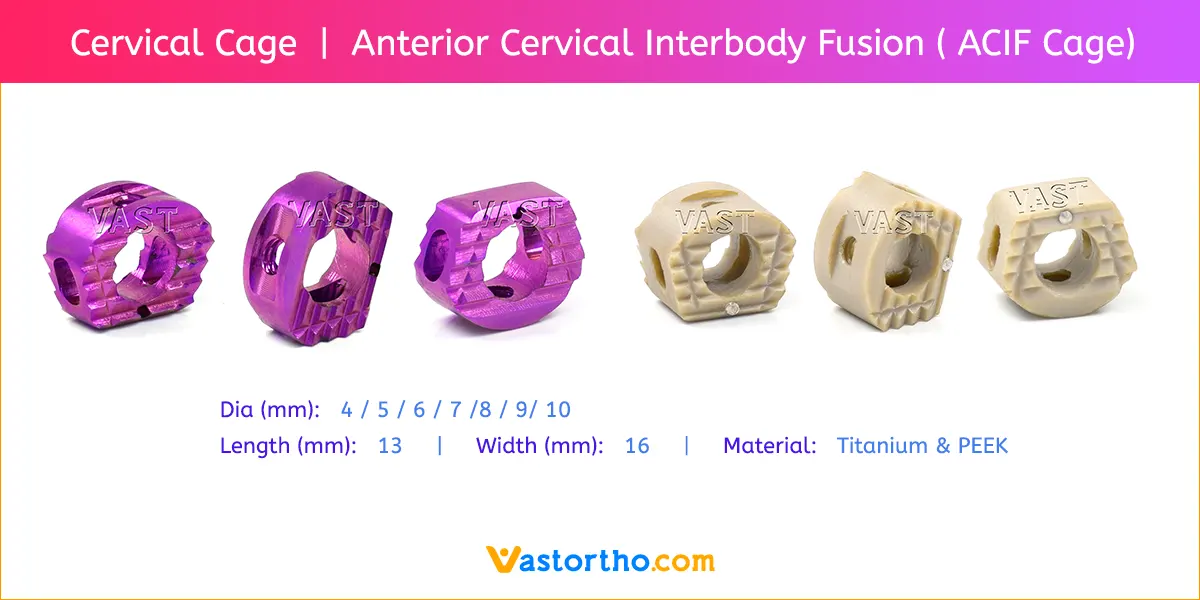
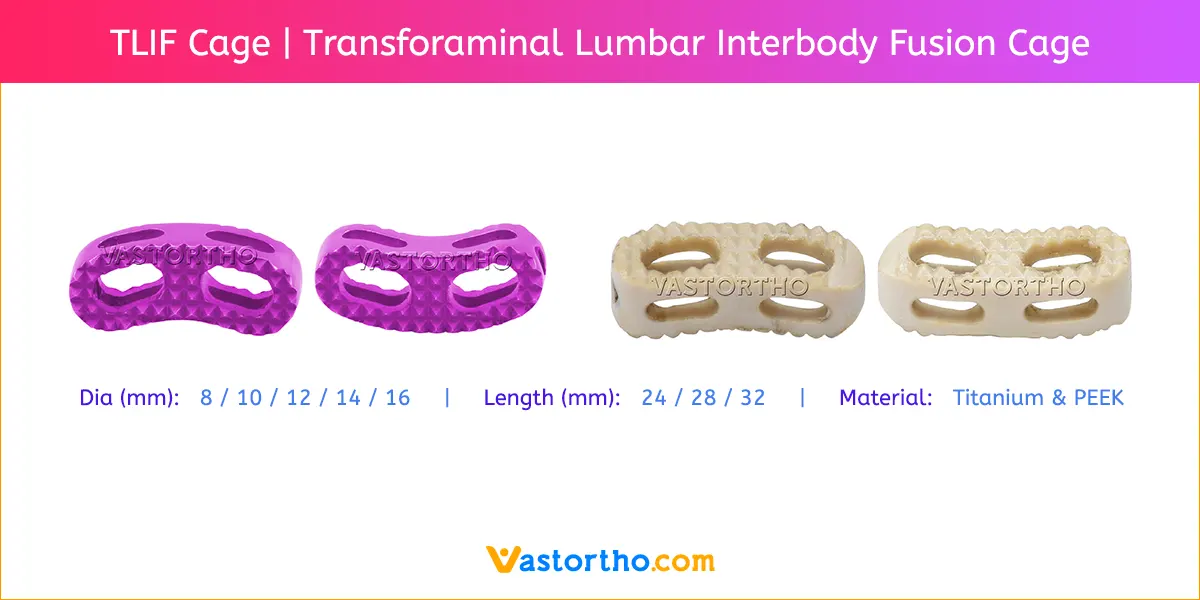
PLIF using local facet joint autograft and pedicle screw fixation
Study design: This is a retrospective study of 42 patients having lumbar degenerative disease or spondylolytic spondylolisthesis treated by posterior lumbar interbody fusion (PLIF) using local autogenous facet joint graft and pedicle screw fixation with an average follow-up time of 8.5 years.
Objectives: To evaluate the radiographic and clinical results of patients treated with PLIF using adjacent facet joint autograft and pedicle screw internal fixation.
Summary of background data: Some goals of spinal surgery have been achieved by interbody arthrodesis using a posterior approach popularized by Cloward. However, significant problems including bone graft collapse, resorption, nonunion, persistent neurologic compression, and iliac crest donor complication using the classic PLIF remain. There are few reports describing the results of a PLIF by total facet joint excision.
Methods: Forty-two patients (average, 53.2 years) treated at our institution with PLIF by total facetectomy were followed for an average period of 8.5 years. The changes in the Japanese Orthopedic Association score, the recovery rate, complications, and radiographic findings were evaluated.
Results: Good radiographic fusion (92.9%) and clinical results (postoperative recovery rate of 76% in the Japanese Orthopedic Association score) were achieved by PLIF using local facet joint autograft and pedicle screw fixation in treating patients with debilitating lumbar degenerative disease. The complications related to the operative procedure occurred in three patients of delayed union.
Conclusions: For lumbar degenerative diseases with osteophytic changes of facet joints, PLIF using pedicle screw fixation and local autogenous bones obtained from facet excision may be justified as a treatment opinion. The procedure as described offers advantages for spinal surgery when PLIF is warranted.