Titanium Mesh Cage Introduction, Sizes, Uses and Advantages.

Titanium Mesh Cage Introduction
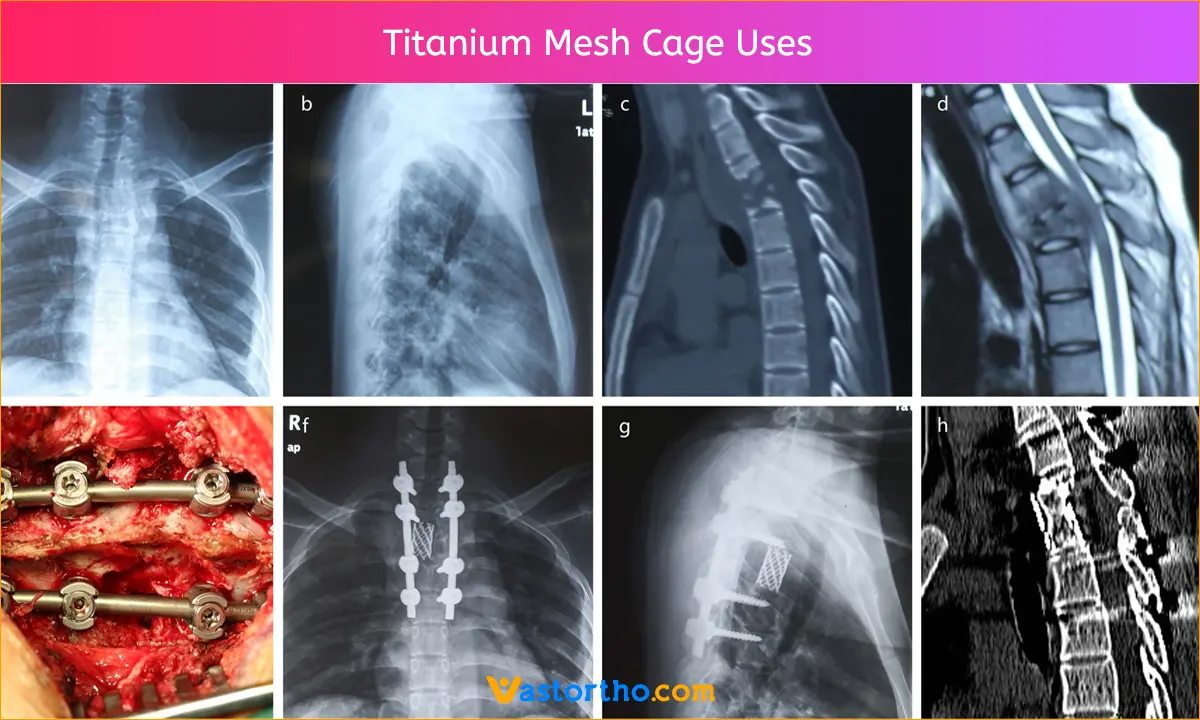
The introduction of the Titanium Mesh Cage in spinal surgery has opened up a variety of applications that are realizable as a result of the versatility of the implant.
Since their debut in 1986, Titanium Mesh Cages have been extensively employed for spine restoration. It is not necessary to harvest huge structural bone grafts because these cages can be employed as structural devices containing autologous local bone or iliac crest bone transplant.
Titanium Mesh Cage Sizes
Titanium of the highest quality, which has exceptional biocompatibility, strength, and durability, is typically used to make Titanium Mesh Cage. Because titanium is radiolucent and does not interfere with X-rays or other imaging methods, the fusion site can be clearly seen after surgery.
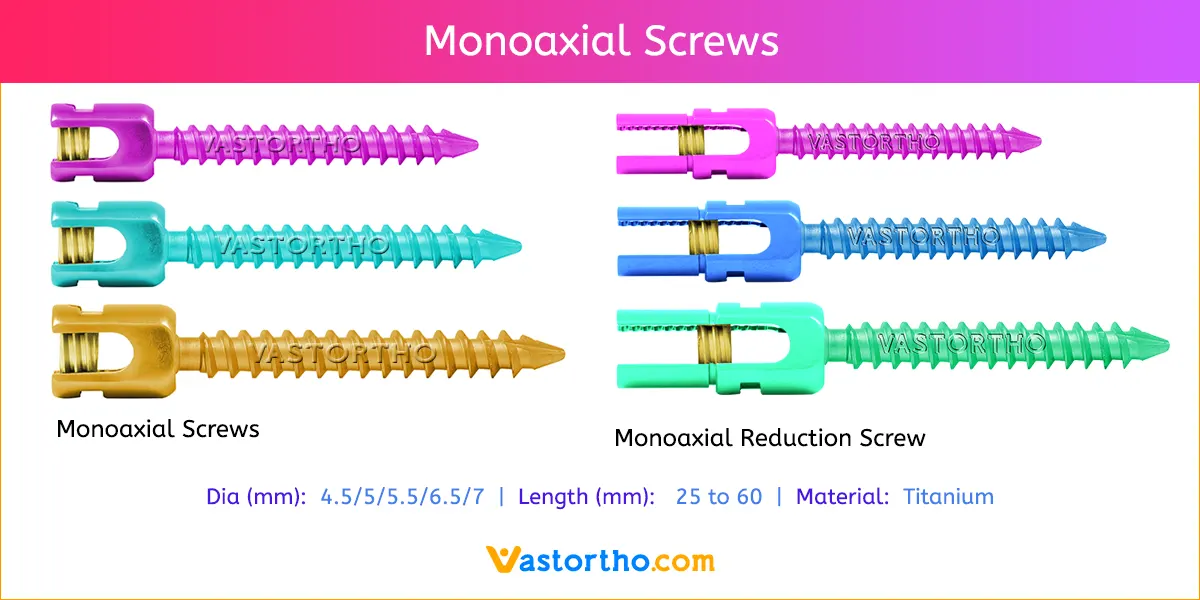
Titanium Mesh Cage are available in various diameters and lengths.
Diameters: 10mm, 12mm, 14mm, 16mm, 18mm, 20mm, 22mm, 24mm and 26mm
Lengths: 60mm, 70mm, 80mm, 90mm and 100mm